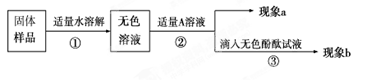
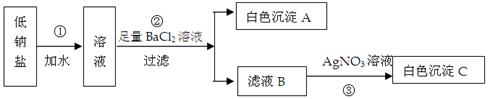
��Ŀ����
��9�֣�СѾ��̽��CO2��NaOH��Һ��Ӧ��ʵ���У����������������ռ���һ��CO2���壬Ȼ��Ѹ�������м���һ������NaOHŨ��Һ�������������ڷ�գ���©����������ҡ�������ޣ����������ܿ�����һ��ʱ��������������¹���������Ӧ�������¶ȵı仯���Բ��ƣ���
�����ۡ�СѾ��Ϊ�����ޱ��˵��CO2��NaOH��Һ������Ӧ��С���˽��۲������������һ����֤����֤������ ��2�֣���������Ϊʲô�ֺ���������Dz��Ƿ�Ӧ�ֲ��������壬������ijɷ֣�
��������롿a��������CO2 b�������� ��
���������ϡ���Na2CO3���н�ǿ�����ȶ��ԣ�ֻ���ڸ��������²Ż�ֽ⣻
�����������ᷴӦ�����������Һ��Ӧ2Al+2NaOH+2H2O==2NaAlO2+3X��
��ʵ����֤��
�ٽ������ڴ�Ѹ����ȼ�ŵ�ľ������ڣ��������ۡ���һ������������ɫ���棬Ƭ�̺�Ϩ��֤������ ������
��ȡ������Ƭ���Թ��У��ȼ���������ˮ�������Ա仯��Ƭ�̺��ټ�����������������Ũ��Һ���������ݡ��ò�ʵ���Ŀ���� ��2�֣���
�����ۡ�CO2��NaOH��Ӧ�Ļ�ѧ����ʽ�� ��
����˼��Ӧ�á�������������������������ʢװ�������ʣ�Ҳ����ʢװ ���ʡ�
�����ۡ�СѾ��Ϊ�����ޱ��˵��CO2��NaOH��Һ������Ӧ��С���˽��۲������������һ����֤����֤������ ��2�֣���������Ϊʲô�ֺ���������Dz��Ƿ�Ӧ�ֲ��������壬������ijɷ֣�
��������롿a��������CO2 b�������� ��
���������ϡ���Na2CO3���н�ǿ�����ȶ��ԣ�ֻ���ڸ��������²Ż�ֽ⣻
�����������ᷴӦ�����������Һ��Ӧ2Al+2NaOH+2H2O==2NaAlO2+3X��
��ʵ����֤��
�ٽ������ڴ�Ѹ����ȼ�ŵ�ľ������ڣ��������ۡ���һ������������ɫ���棬Ƭ�̺�Ϩ��֤������ ������
��ȡ������Ƭ���Թ��У��ȼ���������ˮ�������Ա仯��Ƭ�̺��ټ�����������������Ũ��Һ���������ݡ��ò�ʵ���Ŀ���� ��2�֣���
�����ۡ�CO2��NaOH��Ӧ�Ļ�ѧ����ʽ�� ��
����˼��Ӧ�á�������������������������ʢװ�������ʣ�Ҳ����ʢװ ���ʡ�
����Һ�еμ�ϡ���ᣬ���Ƿ���������� ���� b �Ա����飬֤����������ˮ��Ӧ����������������Һ��Ӧ 2NaOH+CO2 ="=" Na2CO3+ H2O ��������
�����ۡ�����CO2��NaOH��Һ������Ӧ������̼���ƣ����ԣ���֤�����ǣ�ȡ�����������з�Ӧ�����Һ���Թ��У����������ϡ���ᣬ���������������ʹ����ʯ��ˮ����ǣ�˵��ԭCO2���屻NaOH��Һ���գ�
��������롿���������غ㶨�ɽ��в��룬������������
��ʵ����֤������ȼ�յ�����Ŀ�֪����������������֤������b������
����ʵ�����ݺ��̿�֪���ò�ʵ���Ŀ������֤���������Һ��Ӧ��
�����ۡ�CO2��NaOH��ӦӦ�ķ���ʽ�ǣ�CO2+2NaOH=Na2CO3+H2O��
����˼��Ӧ�á������Ͽ�֪�����������ᷴӦ������Ӧ�����ԣ�������������������������ʢװ�������ʣ�Ҳ����ʢװ�������ʣ�
��������롿���������غ㶨�ɽ��в��룬������������
��ʵ����֤������ȼ�յ�����Ŀ�֪����������������֤������b������
����ʵ�����ݺ��̿�֪���ò�ʵ���Ŀ������֤���������Һ��Ӧ��
�����ۡ�CO2��NaOH��ӦӦ�ķ���ʽ�ǣ�CO2+2NaOH=Na2CO3+H2O��
����˼��Ӧ�á������Ͽ�֪�����������ᷴӦ������Ӧ�����ԣ�������������������������ʢװ�������ʣ�Ҳ����ʢװ�������ʣ�

��ϰ��ϵ�д�
�����Ŀ