��Ŀ����
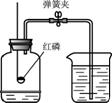
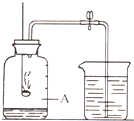 ������һ�ֱ������Ȼ��Դ������ͼ��ʾװ�ã��ⶨ�����������ĺ�����ʵ��ʱ���ڼ���ƿ���������ˮ�����ϼǺţ��õ��ɼмн��齺�ܣ���ȼȼ�ճ��ڵĺ�����������ƿ�в�������������������Ϩ����ֺ�����ʣ�࣬��ȴ�����ɼУ��ձ��е�ˮ����������ƿ�У�Һ��������ͼ��Aλ�ã�
������һ�ֱ������Ȼ��Դ������ͼ��ʾװ�ã��ⶨ�����������ĺ�����ʵ��ʱ���ڼ���ƿ���������ˮ�����ϼǺţ��õ��ɼмн��齺�ܣ���ȼȼ�ճ��ڵĺ�����������ƿ�в�������������������Ϩ����ֺ�����ʣ�࣬��ȴ�����ɼУ��ձ��е�ˮ����������ƿ�У�Һ��������ͼ��Aλ�ã���1�������ڿ�����ȼ��ʱ����
��������
��������
��ͬʱ�ų���������
��������
����Ӧ�����ֱ���ʽΪ����+����
����������
| ��ȼ |
����+����
����������
��| ��ȼ |
��2��������ʵ������ɵó��Ľ�����
����Լռ��������������֮һ
����Լռ��������������֮һ
����3�����ձ��е�ˮ����������ƿ�У�Һ��û��������ͼ��Aλ�ã��������ܵ�ԭ��
��������1�������ڿ������ܹ����ҵ�ȼ�գ��ų��������ȣ����������İ��̣�
��2�����ݽ��뼯��ƿ��ˮ�Ķ��ٿ����жϿ����������ĺ�����
��3��װ��©�������ײ��㣬û����ȴ�����¾ʹ��ɼУ������ܵ���Һ��û��������ͼ��Aλ�ã�
��2�����ݽ��뼯��ƿ��ˮ�Ķ��ٿ����жϿ����������ĺ�����
��3��װ��©�������ײ��㣬û����ȴ�����¾ʹ��ɼУ������ܵ���Һ��û��������ͼ��Aλ�ã�
����⣺��1�������ڿ�����ȼ��ʱ���������İ��̣��ų��������ȣ���Ӧ�����ֱ���ʽΪ������+����
���������ף�
��������İ��̣��������ȣ�����+����
���������ף�
��2�����뼯��ƿ�е�ˮԼռ����ƿ�ݻ������֮һ��˵������Լռ��������������֮һ��
�������Լռ��������������֮һ��
��3�����ܵ�ԭ���У�װ��©�������ײ��㣬û����ȴ�����¾ʹ��ɼУ���Щ��������ܵ���Һ��û��������ͼ��Aλ�ã�
| ��ȼ |
��������İ��̣��������ȣ�����+����
| ��ȼ |
��2�����뼯��ƿ�е�ˮԼռ����ƿ�ݻ������֮һ��˵������Լռ��������������֮һ��
�������Լռ��������������֮һ��
��3�����ܵ�ԭ���У�װ��©�������ײ��㣬û����ȴ�����¾ʹ��ɼУ���Щ��������ܵ���Һ��û��������ͼ��Aλ�ã�
��������ѧʵ�������ǻ�ѧʵ����ͻ�����������IJ��֣�Ҳ�ǽ��з��������ó����۵����ݣ��������ʵ����ʺ��֮��ķ�Ӧ��ϵ����������߹۲�ʵ�顢����ʵ������������ԣ��Ի�ѧʵ�鲻��Ҫ����۲죬��Ӧ�������ʵ�顢�۲�ʵ������ķ�����

��ϰ��ϵ�д�
 ��һ����ͬ���ɽ�����ϵ�д�
��һ����ͬ���ɽ�����ϵ�д� ������Ӧ���ϵ�д�
������Ӧ���ϵ�д� ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�
�����Ŀ