��Ŀ����
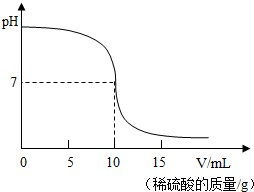 ��2013?�żҸ���ģ�⣩�����кͷ�Ӧ���Բⶨ������Һ�����ʵ�������������ͼΪij�βⶨ�����У���Һ��pH�����μӵ�ij����Һ����ı仯���仯�Ĺ�ϵͼ��
��2013?�żҸ���ģ�⣩�����кͷ�Ӧ���Բⶨ������Һ�����ʵ�������������ͼΪij�βⶨ�����У���Һ��pH�����μӵ�ij����Һ����ı仯���仯�Ĺ�ϵͼ����1��������ͼ���ߣ��жϸ�ʵ������ǽ�
����Һ
����Һ
�μӵ���һ����Һ�У�����ѡ�õ�ָʾ������̪
��̪
����������Һ�еķ�̪�Ӻ�ɫ�պñ����ɫ
��Һ�еķ�̪�Ӻ�ɫ�պñ����ɫ
����ʱ��ֹͣ�μ�������Һ����2�����ʵ����ʹ�õ��������ᣬ�����������ƣ�ȡ����������Һ25g�������м���������������Ϊ9.8%��ϡ���ᣬ����ϡ�������������ҺpH�ı仯�������ͼ��ʾ�����㣺
����ȡ����������Һ�����ʵ�����������
�ڵ�������ȫ��Ӧʱ��������Һ�����ʵ���������������������ȷ��0.1%��
����������ͼ����ʾ��֪����ʼʱ��Һ��pH����7���Լ��ԣ��������Һ�еμ�����Һ��ǡ����ȫ��Ӧʱ����Һ�����ԣ���̪����ɫ�����ݻ�ѧ����ʽ���Խ�����صļ��㣮
����⣺��1��������Һ��pH�仯�����֪����ʼʱ��Һ��pH����7���Լ��ԣ�������Һ��pHС��7���������Һ�еμ�����Һ�����Կ����ǵμ�����ȳ������ᣬ�����п������ü���ʹ��̪����ԭ����Ԥ���ڼ�Һ�еμӷ�̪��Һ������Һ�еķ�̪�Ӻ�ɫ�պñ����ɫ�����кͷ�Ӧǡ����ɣ�
��2�������Һ���ʵ���������Ϊx�����ɵ������Ƶ�����Ϊy��
2NaOH+H2SO4�TNa2SO4+2H2O
80 98 142
25g��x 10g��9.8% y
=
=
x=3.2% y=1.42g
������Һ�����ʵ���������=
��100%=4.1%
�𣺴���Һ���ʵ���������Ϊ3.2%��������Һ�����ʵ���������4.1%��
�ʴ�Ϊ����1������Һ����̪����Һ�еķ�̪�Ӻ�ɫ�պñ����ɫ����2������ȡ����������Һ�����ʵ���������Ϊ3.2%���ڵ�������ȫ��Ӧʱ��������Һ�����ʵ���������Ϊ4.1%��
��2�������Һ���ʵ���������Ϊx�����ɵ������Ƶ�����Ϊy��
2NaOH+H2SO4�TNa2SO4+2H2O
80 98 142
25g��x 10g��9.8% y
| 80 |
| 25g��x |
| 98 |
| 10��9.8% |
| 142 |
| y |
x=3.2% y=1.42g
������Һ�����ʵ���������=
| 1.42g |
| 25g+10g |
�𣺴���Һ���ʵ���������Ϊ3.2%��������Һ�����ʵ���������4.1%��
�ʴ�Ϊ����1������Һ����̪����Һ�еķ�̪�Ӻ�ɫ�պñ����ɫ����2������ȡ����������Һ�����ʵ���������Ϊ3.2%���ڵ�������ȫ��Ӧʱ��������Һ�����ʵ���������Ϊ4.1%��
��������������ĿҪ�ῴ�۵㣬�۵���ָͼ���ڱ仯�����з������˴��ת����Ǹ��㣮���ij�������������������ijһ����������ֹͣ��ɵģ����ͼ���漰��ѧ��Ӧ���۵�ͨ���Ǹ÷�Ӧ�Ľ����㣮���۵�ķ����ؼ���������õ�����ĺ��壮

��ϰ��ϵ�д�
 ȫ�ſ��䵥Ԫ�����������ܸ�ϰϵ�д�
ȫ�ſ��䵥Ԫ�����������ܸ�ϰϵ�д� Ʒѧ˫�ž�ϵ�д�
Ʒѧ˫�ž�ϵ�д� Сѧ��ĩ���100��ϵ�д�
Сѧ��ĩ���100��ϵ�д�
�����Ŀ
 ��2013?�żҸ���ģ�⣩��ͼ��ʾװ�ÿ�����������ռ������顢���Ӻ�����IJ�������ͼʾװ�ò�����ɵ�ʵ���ǣ�������
��2013?�żҸ���ģ�⣩��ͼ��ʾװ�ÿ�����������ռ������顢���Ӻ�����IJ�������ͼʾװ�ò�����ɵ�ʵ���ǣ�������