��Ŀ����
����������ѧ����ͬѧ����ѧ���Ļ�ѧ֪ʶ����������е����⣺
��1����ϡ���ᡢ��ʯ�ҡ�ʳ�Ρ��������ͭ���������ѡ����ʵ����ʣ����仯ѧʽ�������пո��У��ɼ���ƾ����Ƿ���ˮ����
��2����Һ�����ȳ���
��3������������������Һ���Թ��У����뼸�η�̪��Һ����Һ��
��4��Ϊ��ȥ�����ϵ��⼣��ijѧ�����������������������������У������ȿ����⼣�ܽ⣬��Һ��
��1����ϡ���ᡢ��ʯ�ҡ�ʳ�Ρ��������ͭ���������ѡ����ʵ����ʣ����仯ѧʽ�������пո��У��ɼ���ƾ����Ƿ���ˮ����
CuSO4
CuSO4
�����������Ϸʵ���KNO3
KNO3
�������ڳ�ȥ�����������������H2SO4
H2SO4
������ʳƷ��ζ������NaCl
NaCl
�������������ϵ���Ca��OH��2
Ca��OH��2
����������ϴ�Ӽ�����Na2CO3
Na2CO3
����2����Һ�����ȳ���
pH
pH
����ʾ�����ķ�Χ��0��14
0��14
֮�䣬�ⶨ�������㷽������pH��ֽ
pH��ֽ
��ij������PHΪ5��������������
��
�ԣ�ũҵ�ϳ�����ʯ��
��ʯ��
������������������3������������������Һ���Թ��У����뼸�η�̪��Һ����Һ��
��
��
ɫ���������ؼ���ϡ���ᣬ������������Һ�պó���ɫʱ����Һ����
��
�ԣ��˷�Ӧ��ѧ����ʽ��NaOH+HCl�TNaCl+H2O
NaOH+HCl�TNaCl+H2O
�����������ֽⷴӦ
���ֽⷴӦ
���������Ӧ���ͣ�����4��Ϊ��ȥ�����ϵ��⼣��ijѧ�����������������������������У������ȿ����⼣�ܽ⣬��Һ��
��
��
ɫ��������Ϊ���û�ѧ����ʽ��ʾ��Fe2O3+6HCl�T2FeCl3+3H2O
Fe2O3+6HCl�T2FeCl3+3H2O
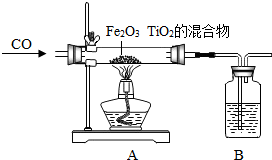
����һ������ֿ�����������������
����
������������Ϊ���û�ѧ����ʽ��ʾ��Fe+2HCl�TFeCl2+H2��
Fe+2HCl�TFeCl2+H2��
����������1���������ʵ����ʾ�����;����
��2��������Һ���ȵIJ�������������������ԵĹ�ϵ����
��3�������������������Ʒ����кͷ�Ӧ�Ĺ��̷���
��4����������Ļ�ѧ���ʷ���
��2��������Һ���ȵIJ�������������������ԵĹ�ϵ����
��3�������������������Ʒ����кͷ�Ӧ�Ĺ��̷���
��4����������Ļ�ѧ���ʷ���
����⣺��1����ɫ����ͭ��ĩ��ˮ�ܱ�������˿ɼ���ƾ����Ƿ���ˮ��������к��е�Ԫ�غͼ�Ԫ�أ����ڸ��Ϸʣ�����������������ﷴӦ�������ڳ�ȥ��������������Ȼ��ƿ���ʳƷ��ζ����ʯ�ҽ�������Ĩǽ�������������ϣ�̼������Һ�ʼ��ԣ��ɳ����ۣ��ʿ�������ϴ�Ӽ������ݻ��ϼۼ���д��ѧʽ��ԭ��ֱ�д�����ǵĻ�ѧʽΪ
��1��CuSO4�� KNO3�� H2SO4�� NaCl�� Ca��OH��2�� Na2CO3
��2����Һ�����ȳ��� pH����ʾ�����ķ�Χ�� 0��14֮�䣬�ⶨ�������㷽������ pH��ֽ��PHС��7�����ԣ���pHΪ5������������ ���ԣ�ũҵ�ϳ��� ��ʯ������������������
��3������������Һ��ʹ��̪��Һ�� ��ɫ���������������ᷴӦ������������������ǡ����ȫ��Ӧʱ��ҺΪ�Ȼ�����Һ�������ԣ��˷�Ӧ��ѧ����ʽ�� NaOH+HCl�TNaCl+H2O���÷�Ӧ�����ֻ����サ���ɷ������������ֻ�����ķ�Ӧ������ ���ֽⷴӦ
��4����������������Ӧ�����Ȼ�����ˮ���Ȼ�����ҺΪ��ɫ����ѧ����ʽΪ Fe2O3+6HCl�T2FeCl3+3H2O��������ȫ��Ӧ���������������Ӧ������������˿������������ɣ���ѧ����ʽΪ Fe+2HCl�TFeCl2+H2��
�ʴ�Ϊ����1��CuSO4�� KNO3�� H2SO4�� NaCl�� Ca��OH��2�� Na2CO3��
��2��pH 0��14 pH��ֽ�� �� ��ʯ�ң�
��3���� �� NaOH+HCl�TNaCl+H2O�����ֽⷴӦ
��4���� Fe2O3+6HCl�T2FeCl3+3H2O������ Fe+2HCl�TFeCl2+H2��
��1��CuSO4�� KNO3�� H2SO4�� NaCl�� Ca��OH��2�� Na2CO3
��2����Һ�����ȳ��� pH����ʾ�����ķ�Χ�� 0��14֮�䣬�ⶨ�������㷽������ pH��ֽ��PHС��7�����ԣ���pHΪ5������������ ���ԣ�ũҵ�ϳ��� ��ʯ������������������
��3������������Һ��ʹ��̪��Һ�� ��ɫ���������������ᷴӦ������������������ǡ����ȫ��Ӧʱ��ҺΪ�Ȼ�����Һ�������ԣ��˷�Ӧ��ѧ����ʽ�� NaOH+HCl�TNaCl+H2O���÷�Ӧ�����ֻ����サ���ɷ������������ֻ�����ķ�Ӧ������ ���ֽⷴӦ
��4����������������Ӧ�����Ȼ�����ˮ���Ȼ�����ҺΪ��ɫ����ѧ����ʽΪ Fe2O3+6HCl�T2FeCl3+3H2O��������ȫ��Ӧ���������������Ӧ������������˿������������ɣ���ѧ����ʽΪ Fe+2HCl�TFeCl2+H2��
�ʴ�Ϊ����1��CuSO4�� KNO3�� H2SO4�� NaCl�� Ca��OH��2�� Na2CO3��
��2��pH 0��14 pH��ֽ�� �� ��ʯ�ң�
��3���� �� NaOH+HCl�TNaCl+H2O�����ֽⷴӦ
��4���� Fe2O3+6HCl�T2FeCl3+3H2O������ Fe+2HCl�TFeCl2+H2��
���������⿼��������еĻ���֪ʶ��Ӧ�������գ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ���϶�һ�š����ҹ��������ơ��ɹ�����ĵ�һ������̽���������϶�һ�š��ijɹ����䣬ʹȫ�����������裮��ͬѧ���Ķ����ж��IJ��ش��й����⣮
���϶�һ�š����ҹ��������ơ��ɹ�����ĵ�һ������̽���������϶�һ�š��ijɹ����䣬ʹȫ�����������裮��ͬѧ���Ķ����ж��IJ��ش��й����⣮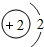 D�����ڽ���Ԫ�أ��ڻ�ѧ��Ӧ���õ�����
D�����ڽ���Ԫ�أ��ڻ�ѧ��Ӧ���õ�����