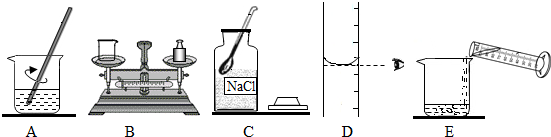
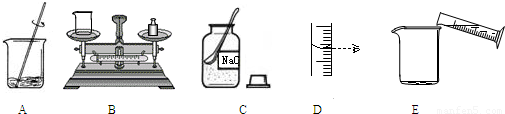
��Ŀ����
Ϊ�˳�ȥNaCl �е�����RCl2����Ҫ����10%������������Һ�������²������ش���������

��1������������������Һʱ�����в�������ȷ�IJ���˳����______������ĸ��ţ�
��2���ڳ�ȥNaCl �����л��е�RCl2ʱ��ȡ20�˸û�������100��ˮ�У���ȫ�ܽ⣬�ټ���80����õ�����������Һ��ǡ�ò��ٲ�����������ˣ���9.8��������
��д����Ӧ�Ļ�ѧ����ʽ______
��������֪������ⷴӦ�����Ȼ���������x���ı���ʽ______
��R��Ԫ�ص����ԭ��������______
����������Һ�м���173.8��ˮ��������Һ����������Ϊ______��
�⣺��1������һ��������������������������Һʱ����Ҫ��֪���������������ƶ��ٿˣ��ܼ�ˮ���ٿˣ��������Ǽ��㣮��������ȳƹ���ʳ�Σ�����ȡˮ��������ܽ⣬�ʲ���Ϊ�����㣬��������ȡ���ܽ⣮�ʴ�Ϊ��C��B��D��E��A��
��2��80g10%���������Ƶ�����=80g��10%=8g
�����ɵ��Ȼ��Ƶ�����Ϊx��R�����ԭ��������Ϊy
RCl2+2NaOH=R��OH��2+2NaCl
80 34+y 117
8g 9.8g x

x=11.7g

y=64
��μӷ�Ӧ��RCl2����ΪZ
RCl2+2NaOH=R��OH��2+2NaCl
135 80
Z 8g

z=13.5g
��Ӧ��������Һ����������= ��100%=5%
��100%=5%
��������Һ����������Ϊ5%��
��������1������Ҫ��������������һ������һ��������������Һ�IJ��裬�������㣬��������ȡ���ܽ⣬װƿ��
��2�����ݲμӷ�Ӧ���������Ƶ��������Ϳɼ�������ɵ��Ȼ��Ƶ�������Ȼ�����û�ѧ����ʽ���������Ƶ��������������������г�����ʽ���Ϳɼ����R�����ԭ����������Ӧ��������Һ�����ʵ�����=�����������Ȼ�������+��Ӧ�����Ȼ��Ƶ���������Ӧ��������Һ����=������������+ˮ������+������������-������������Ȼ�����������������= ��100%���㼴�ɣ�
��100%���㼴�ɣ�
������������Ҫ����ѧ�����û�ѧ����ʽ����������������ʽ�ۺϷ����ͽ��ʵ�������������������ѧ�����������˼ά��ȣ�ǿ����ѧ������֪ʶ��������
��2��80g10%���������Ƶ�����=80g��10%=8g
�����ɵ��Ȼ��Ƶ�����Ϊx��R�����ԭ��������Ϊy
RCl2+2NaOH=R��OH��2+2NaCl
80 34+y 117
8g 9.8g x

x=11.7g

y=64
��μӷ�Ӧ��RCl2����ΪZ
RCl2+2NaOH=R��OH��2+2NaCl
135 80
Z 8g

z=13.5g
��Ӧ��������Һ����������=
 ��100%=5%
��100%=5%��������Һ����������Ϊ5%��
��������1������Ҫ��������������һ������һ��������������Һ�IJ��裬�������㣬��������ȡ���ܽ⣬װƿ��
��2�����ݲμӷ�Ӧ���������Ƶ��������Ϳɼ�������ɵ��Ȼ��Ƶ�������Ȼ�����û�ѧ����ʽ���������Ƶ��������������������г�����ʽ���Ϳɼ����R�����ԭ����������Ӧ��������Һ�����ʵ�����=�����������Ȼ�������+��Ӧ�����Ȼ��Ƶ���������Ӧ��������Һ����=������������+ˮ������+������������-������������Ȼ�����������������=
 ��100%���㼴�ɣ�
��100%���㼴�ɣ�������������Ҫ����ѧ�����û�ѧ����ʽ����������������ʽ�ۺϷ����ͽ��ʵ�������������������ѧ�����������˼ά��ȣ�ǿ����ѧ������֪ʶ��������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ