��Ŀ����
���û�ѧ����ʽ��ʾ��������Ҫ��ķ�Ӧ
��1������ʯ��ʯ��
��2����˿��������ȼ�գ�
��3��ͭ����������Һ�ķ�Ӧ��
��4������������ϡ������кͷ�Ӧ��
��5������ʯ�ң�
��6����ҵ���������ƣ�
��7�����������������
��8��˫��ˮ��������
��9�����������̼��
��10����ҵ������
��1������ʯ��ʯ��
CaCO3
CaO+CO2��
| ||
CaCO3
CaO+CO2��
��
| ||
��2����˿��������ȼ�գ�
3Fe+2O2
Fe3O4
| ||
3Fe+2O2
Fe3O4
��
| ||
��3��ͭ����������Һ�ķ�Ӧ��
Cu+2AgNO3�TCu��NO3��2+2Ag
Cu+2AgNO3�TCu��NO3��2+2Ag
����4������������ϡ������кͷ�Ӧ��
Ca��OH��2+2HCl�TCaCl2+2H2O
Ca��OH��2+2HCl�TCaCl2+2H2O
����5������ʯ�ң�
CaO+H2O�TCa��OH��2
CaO+H2O�TCa��OH��2
����6����ҵ���������ƣ�
Ca��OH��2+Na2CO3�TCaCO3��+2NaOH
Ca��OH��2+Na2CO3�TCaCO3��+2NaOH
����7�����������������
2KMnO4
K2MnO4+MnO2+O2��
| ||
2KMnO4
K2MnO4+MnO2+O2��
��
| ||
��8��˫��ˮ��������
2H2O2
2H2O+O2��
| ||
2H2O2
2H2O+O2��
��
| ||
��9�����������̼��
CO2+Ca��OH��2�TCaCO3��+H2O
CO2+Ca��OH��2�TCaCO3��+H2O
����10����ҵ������
Fe2O3+3CO
2Fe+3CO2
| ||
Fe2O3+3CO
2Fe+3CO2
��
| ||
���������ȸ��ݷ�Ӧԭ���ҳ���Ӧ��������Ӧ���������ݻ�ѧ����ʽ����д���������������д���ɣ�
����⣺��1������������ʯ��ʯ���������ƺͶ�����̼����Ӧ�Ļ�ѧ����ʽΪ��CaCO3
CaO+CO2����
��2������������ȼ��������������������Ӧ�Ļ�ѧ����ʽΪ��3Fe+2O2
Fe3O4��
��3��ͭ����������Ӧ������������ͭ����Ӧ�Ļ�ѧ����ʽΪ��Cu+2AgNO3�TCu��NO3��2+2Ag��
��4������������ϡ���ᷴӦ�����Ȼ��ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪ��Ca��OH��2+2HCl�TCaCl2+2H2O��
��5����������ˮ��Ӧ�����������ƣ���ʯ�ң�����Ӧ�Ļ�ѧ����ʽΪ��CaO+H2O�TCa��OH��2��
��6����ҵ���������Ʋ��õ���̼������Һ������������Һ��Ӧ����̼��Ƴ������������ƣ���Ӧ�Ļ�ѧ����ʽΪ��Ca��OH��2+Na2CO3�TCaCO3��+2NaOH��
��7����������ڼ�����������������ء��������̺���������Ӧ�Ļ�ѧ����ʽ��2KMnO4
K2MnO4+MnO2+O2����
��8��˫��ˮ�ڶ������̵Ĵ�����������ˮ����������Ӧ�Ļ�ѧ����ʽΪ��2H2O2
2H2O+O2����
��9�����������̼���ó����ʯ��ˮ��������̼���������Ʒ�Ӧ����̼��Ƴ�����ˮ����Ӧ�Ļ�ѧ����ʽΪ��CO2+Ca��OH��2�TCaCO3��+H2O��
��10����ҵ����һ����̼��ԭ��������������Ҫ������CO�Ļ�ԭ�ԣ��ڸ����º���������Ӧ�������Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪFe2O3+3CO
2Fe+3CO2��
�ʴ�Ϊ����1��CaCO3
CaO+CO2������2��3Fe+2O2
Fe3O4����3��Cu+2AgNO3�TCu��NO3��2+2Ag����4��Ca��OH��2+2HCl�TCaCl2+2H2O����5��CaO+H2O�TCa��OH��2����6��Ca��OH��2+Na2CO3�TCaCO3��+2NaOH����7��2KMnO4
K2MnO4+MnO2+O2������8��2H2O2
2H2O+O2������9��CO2+Ca��OH��2�TCaCO3��+H2O����10��Fe2O3+3CO
2Fe+3CO2��
| ||
��2������������ȼ��������������������Ӧ�Ļ�ѧ����ʽΪ��3Fe+2O2
| ||
��3��ͭ����������Ӧ������������ͭ����Ӧ�Ļ�ѧ����ʽΪ��Cu+2AgNO3�TCu��NO3��2+2Ag��
��4������������ϡ���ᷴӦ�����Ȼ��ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪ��Ca��OH��2+2HCl�TCaCl2+2H2O��
��5����������ˮ��Ӧ�����������ƣ���ʯ�ң�����Ӧ�Ļ�ѧ����ʽΪ��CaO+H2O�TCa��OH��2��
��6����ҵ���������Ʋ��õ���̼������Һ������������Һ��Ӧ����̼��Ƴ������������ƣ���Ӧ�Ļ�ѧ����ʽΪ��Ca��OH��2+Na2CO3�TCaCO3��+2NaOH��
��7����������ڼ�����������������ء��������̺���������Ӧ�Ļ�ѧ����ʽ��2KMnO4
| ||
��8��˫��ˮ�ڶ������̵Ĵ�����������ˮ����������Ӧ�Ļ�ѧ����ʽΪ��2H2O2
| ||
��9�����������̼���ó����ʯ��ˮ��������̼���������Ʒ�Ӧ����̼��Ƴ�����ˮ����Ӧ�Ļ�ѧ����ʽΪ��CO2+Ca��OH��2�TCaCO3��+H2O��
��10����ҵ����һ����̼��ԭ��������������Ҫ������CO�Ļ�ԭ�ԣ��ڸ����º���������Ӧ�������Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪFe2O3+3CO
| ||
�ʴ�Ϊ����1��CaCO3
| ||
| ||
| ||
| ||
| ||
�����������ѶȲ�����ѧ�����ݷ�Ӧԭ����д��ѧ����ʽ����������ѧ����ʽ��д�������ֵĴ����в����Ͽ���ʵ�������������غ㶨�ɡ���д������������ŵȣ�

��ϰ��ϵ�д�
 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�
�����Ŀ
��ѧ�����ǻ�ѧ�����ԣ����ܱ�ʾ������ɡ��ṹ���仯�����ʵȣ���ѧ�û�ѧ����Ҫ���ߡ�
��1���û�ѧ���Ż�������ա�
| �� �� | ���� | �������� | ̼��� | |
| �� �� | FeSO4 |
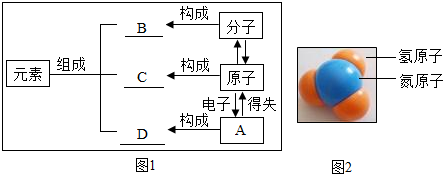
��2����ͼ�ǽ̲������ø���ͼ��֪ʶ���е���������ֱ�д����������A����Ӧ����B��C��D���Ļ�ѧʽ��

��3����ͼ��ij���ʷ���ģ�ͣ����Ļ�ѧʽΪ ��
CuSO4�� ���ɵĻ��������ӡ�ԭ�ӻ����ӣ���
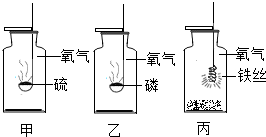
��4���ס��ҡ���������ʵ���ʾ��ͼ��������ͼ���������Ļ�ѧ��Ӧ����ѧ��Ӧԭ����

�� �� ��
д���ס��ҡ�����Ӧ�Ļ�ѧ����ʽ
�� ���� �ҡ� ��