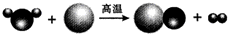��Ŀ����
Ŀǰ������ʹ�õ�ȼ�ϴ�����Ի�ʯȼ�ϣ���ú��ʯ�ͺ���Ȼ���ȣ�
��1����ʯȼ����______��Դ��ѡ�����������������������
��2���������м��������Ҵ���Ϊ����ȼ�ϣ����ʵ���ʡʯ����Դ������һ���̶��ϼ�������β������Ⱦ���Ҵ���ȫȼ�յĻ�ѧ����ʽΪ______��
��3��úȼ��ʱ�ŷų��������壬����������������______��ѡ����ĸ����
A��һ����̼ B��������̼ C���������� D����������
��4��Ϊ���ú�������ʲ�������Ⱦ������һ�������½�úת��Ϊ��ȼ�����壮��ͼ��̼��ˮ�����ڸ����·�Ӧ����ʾ��ͼ���÷�Ӧ�Ļ�ѧ����ʽΪ______��̼�ڸ÷�Ӧ�б��ֳ�______�ԣ�

��1����ʯȼ����______��Դ��ѡ�����������������������
��2���������м��������Ҵ���Ϊ����ȼ�ϣ����ʵ���ʡʯ����Դ������һ���̶��ϼ�������β������Ⱦ���Ҵ���ȫȼ�յĻ�ѧ����ʽΪ______��
��3��úȼ��ʱ�ŷų��������壬����������������______��ѡ����ĸ����
A��һ����̼ B��������̼ C���������� D����������
��4��Ϊ���ú�������ʲ�������Ⱦ������һ�������½�úת��Ϊ��ȼ�����壮��ͼ��̼��ˮ�����ڸ����·�Ӧ����ʾ��ͼ���÷�Ӧ�Ļ�ѧ����ʽΪ______��̼�ڸ÷�Ӧ�б��ֳ�______�ԣ�

��1����ʯȼ���Dz���������Դ���������������
��2���Ҵ���ȫȼ�������ɶ�����̼��ˮ�����C2H5OH+3O2
2CO2+3H2O��
��3��úȼ��ʱ�����Ķ�������͵�������������ˮ���䵽���棬���γ����꣬�۲�ѡ����CD��
��4��������ʾ��ͼ���Կ������ڸ����£�̼��ˮ������Ӧ������һ����̼���������÷�Ӧ��̼���˻�ԭ���ã����C+H2O
CO+H2����ԭ��
��2���Ҵ���ȫȼ�������ɶ�����̼��ˮ�����C2H5OH+3O2
| ||
��3��úȼ��ʱ�����Ķ�������͵�������������ˮ���䵽���棬���γ����꣬�۲�ѡ����CD��
��4��������ʾ��ͼ���Կ������ڸ����£�̼��ˮ������Ӧ������һ����̼���������÷�Ӧ��̼���˻�ԭ���ã����C+H2O
| ||

��ϰ��ϵ�д�
�����Ŀ