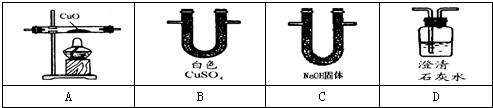
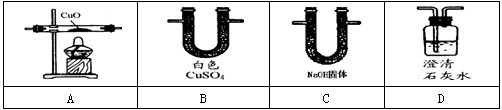
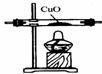
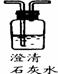
��Ŀ����
����ͼ��ʵ�����ṩ����ʢ��ҩƷ���������ɼ�������ʵ��С�����֤��ijһ��������к��ж�����̼��һ����̼��ˮ��������������ͨ��װ��ʱ������ȫ���ջ�Ӧ������1�����飺�ֱ�֤����������к���ijһ�����壮
��֤�����к���ˮ������ѡ��
��֤�����к��ж�����̼��ѡ��
��ѡ���Ҫ��������ÿ��ֻ��һ�Σ�֤�����к���һ����̼��������ͨ�����Ⱥ�˳��������
��һ����̼��ԭ����ͭ�Ļ�ѧ����ʽ��
��2�����飺�����������������ظ�ʹ�ã���װһ��װ�ã�ͨ��һ��ʵ��֤����������к��ж�����̼��һ����̼��ˮ������
�ٰ�����ͨ�����Ⱥ�˳�����ӵ�����������
�ڴӻ��������Ƕȳ�������ʵ���������β���Ĵ���������
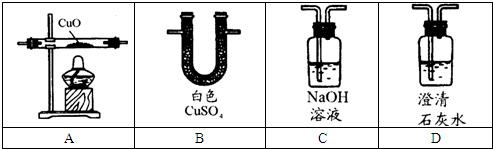
��������1����֤��ˮ����������ˮ����ͭ����֤��������̼�Ĵ��ڣ����ó����ʯ��ˮ��
��֤��CO�Ĵ��ڣ�Ҫ���ų�CO2�ĸ��ţ����û�ԭ����ͭ��ʵ��ʹ����ת��ΪCO2����֤��CO2�Ĵ��ڣ�˵��ԭ��������CO����һ����̼��ԭ����ͭ����ͭ�Ͷ�����̼��
��2�����������ظ�ʹ�ã�ͨ��һ��ʵ��֤������������ɣ������˳��ӦΪˮ������������̼��һ����̼�����ߵ�ʵ�鶼�п�������ˮ�������ټ���һ����̼֮ǰ���ų�������̼�ĸ��ţ���ʼ���ֳ����������г�������ʱ��˵��������̼��C����ȫ��Ӧ���������������Ϊһ����̼��
��֤��CO�Ĵ��ڣ�Ҫ���ų�CO2�ĸ��ţ����û�ԭ����ͭ��ʵ��ʹ����ת��ΪCO2����֤��CO2�Ĵ��ڣ�˵��ԭ��������CO����һ����̼��ԭ����ͭ����ͭ�Ͷ�����̼��
��2�����������ظ�ʹ�ã�ͨ��һ��ʵ��֤������������ɣ������˳��ӦΪˮ������������̼��һ����̼�����ߵ�ʵ�鶼�п�������ˮ�������ټ���һ����̼֮ǰ���ų�������̼�ĸ��ţ���ʼ���ֳ����������г�������ʱ��˵��������̼��C����ȫ��Ӧ���������������Ϊһ����̼��
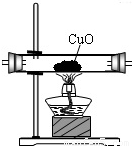
����⣺��ˮ����ͨ����ˮ����ͭ��������ɫ�а�ɫ��Ϊ��ɫ��
�ڶ�����̼ͨ������ʯ��ˮ�У�����ʯ��ˮ����ǣ�
������ͨ������������Һ��ȥ������̼�ų�CO2�ĸ��ţ����û�ԭ����ͭ��ʵ��ʹ����ת��ΪCO2����ͨ�����ʯ��ˮ������ʯ��ˮ�����֤��CO2�Ĵ��ڣ�˵��ԭ��������CO����һ����̼��ԭ����ͭ����ͭ�Ͷ�����̼��
��2�����������ظ�ʹ�ã�ͨ��һ��ʵ��֤������������ɣ������˳��ӦΪˮ������������̼��һ����̼����ͨ���ɫ����ͭ����ˮ��������ͨ�����ʯ��ˮ���������̼���ټ���һ����̼֮ǰ�ų�������̼�ĸ��ţ���ʼ���ֳ����������г�������ʱ��˵��������̼��C����ȫ��Ӧ����������û�ԭ����ͭ��ʵ��ʹ����ת��ΪCO2����ͨ�����ʯ�ң�����һ����̼��
�����������Ϊһ����̼��ȼ�ջ����ô������ռ��ȷ���������
�ʴ�Ϊ��
��1����B�����ɫ����ͭ��ĩ������ɫ��ĩ�����ɫ��
��D�������ʯ��ˮ����Ca��OH��2+CO2�TCaCO3��+H2O��
��C��A��D��
��CO+CuO
Cu+CO2
��2����B��D��A��D ��B��D��C��A��D
��ȼ�ջ����ô������ռ���
�ڶ�����̼ͨ������ʯ��ˮ�У�����ʯ��ˮ����ǣ�
������ͨ������������Һ��ȥ������̼�ų�CO2�ĸ��ţ����û�ԭ����ͭ��ʵ��ʹ����ת��ΪCO2����ͨ�����ʯ��ˮ������ʯ��ˮ�����֤��CO2�Ĵ��ڣ�˵��ԭ��������CO����һ����̼��ԭ����ͭ����ͭ�Ͷ�����̼��
��2�����������ظ�ʹ�ã�ͨ��һ��ʵ��֤������������ɣ������˳��ӦΪˮ������������̼��һ����̼����ͨ���ɫ����ͭ����ˮ��������ͨ�����ʯ��ˮ���������̼���ټ���һ����̼֮ǰ�ų�������̼�ĸ��ţ���ʼ���ֳ����������г�������ʱ��˵��������̼��C����ȫ��Ӧ����������û�ԭ����ͭ��ʵ��ʹ����ת��ΪCO2����ͨ�����ʯ�ң�����һ����̼��
�����������Ϊһ����̼��ȼ�ջ����ô������ռ��ȷ���������
�ʴ�Ϊ��
��1����B�����ɫ����ͭ��ĩ������ɫ��ĩ�����ɫ��
��D�������ʯ��ˮ����Ca��OH��2+CO2�TCaCO3��+H2O��
��C��A��D��
��CO+CuO
| ||
��2����B��D��A��D ��B��D��C��A��D
��ȼ�ջ����ô������ռ���
���������ʵļ���Ҫ�������ʵ����ʣ����ڼ���ʱҪע���ų��֮��ĸ��Ż�������ĸ������أ���Ҫ�����������Ե����ʣ�

��ϰ��ϵ�д�
�����Ŀ
���ᣨH2C2O4����һ�ֶ�Ԫ�ᣬ��һ�����������Ȼᷢ���ֽ⣬ijʵ��С��Բ���ֽ����Ŀ�����Ͻ����˶��ּ��裺��H2��CO����H2��CO2����H2O��CO����H2O��CO2����H2O��CO��CO2��
��1������Щ�����У��������ó��л�ѧ��ѧ�����֪ʶ��ͨ��һ����������ֱ���ų�����___________________�����ţ�������������Ҫͨ��ʵ�������֤��
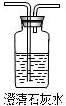
��2������ͼ��ʵ�����ṩ����ʢ��ҩƷ����������֤����ֽ�Ŀ��ܲ��
|
|
|
|
|
| A | B | C | D |
�� ֤�����к���ˮ���������ֵ�ʵ�������� ��
�� ֤�����к��ж�����̼����Ӧ�Ļ�ѧ����ʽ�� ��
�� �����������������ظ�ʹ�ã���װһ��װ�ã�ͨ��һ��ʵ����֤����ֽ�Ŀ��ܲ��������ͨ�����Ⱥ�˳�����ӵ����������� ����A��B��C��D��ʾ����
�ܴӻ��������Ƕȳ�������ʵ���������β���Ĵ���������____________ ��
���ᣨH2C2O4����һ�ֶ�Ԫ�ᣬ��һ�����������Ȼᷢ���ֽ⣬ijʵ��С��Բ���ֽ����Ŀ�����Ͻ����˶��ּ��裺��H2��CO����H2��CO2����H2O��CO����H2O��CO2����H2O��CO��CO2��
��1������Щ�����У��������ó��л�ѧ��ѧ�����֪ʶ��ͨ��һ����������ֱ���ų�����______�����ţ�������������Ҫͨ��ʵ�������֤��
��2������ͼ��ʵ�����ṩ����ʢ��ҩƷ����������֤����ֽ�Ŀ��ܲ��
��֤�����к���ˮ���������ֵ�ʵ��������______��
��֤�����к��ж�����̼����Ӧ�Ļ�ѧ����ʽ��______��
�������������������ظ�ʹ�ã���װһ��װ�ã�ͨ��һ��ʵ����֤����ֽ�Ŀ��ܲ��������ͨ�����Ⱥ�˳�����ӵ�����������______����A��B��C��D��ʾ����
�ܴӻ��������Ƕȳ�������ʵ���������β���Ĵ���������______��
��1������Щ�����У��������ó��л�ѧ��ѧ�����֪ʶ��ͨ��һ����������ֱ���ų�����______�����ţ�������������Ҫͨ��ʵ�������֤��
��2������ͼ��ʵ�����ṩ����ʢ��ҩƷ����������֤����ֽ�Ŀ��ܲ��
 |  |  |  |
| A | B | C | D |
��֤�����к��ж�����̼����Ӧ�Ļ�ѧ����ʽ��______��
�������������������ظ�ʹ�ã���װһ��װ�ã�ͨ��һ��ʵ����֤����ֽ�Ŀ��ܲ��������ͨ�����Ⱥ�˳�����ӵ�����������______����A��B��C��D��ʾ����
�ܴӻ��������Ƕȳ�������ʵ���������β���Ĵ���������______��
���ᣨH2C2O4����һ�ֶ�Ԫ�ᣬ��һ�����������Ȼᷢ���ֽ⣬ijʵ��С��Բ���ֽ����Ŀ�����Ͻ����˶��ּ��裺��H2��CO����H2��CO2����H2O��CO����H2O��CO2����H2O��CO��CO2��
��1������Щ�����У��������ó��л�ѧ��ѧ�����֪ʶ��ͨ��һ����������ֱ���ų�����______�����ţ�������������Ҫͨ��ʵ�������֤��
��2������ͼ��ʵ�����ṩ����ʢ��ҩƷ����������֤����ֽ�Ŀ��ܲ��
��֤�����к���ˮ���������ֵ�ʵ��������______��
��֤�����к��ж�����̼����Ӧ�Ļ�ѧ����ʽ��______��
�������������������ظ�ʹ�ã���װһ��װ�ã�ͨ��һ��ʵ����֤����ֽ�Ŀ��ܲ��������ͨ�����Ⱥ�˳�����ӵ�����������______����A��B��C��D��ʾ����
�ܴӻ��������Ƕȳ�������ʵ���������β���Ĵ���������______��
��1������Щ�����У��������ó��л�ѧ��ѧ�����֪ʶ��ͨ��һ����������ֱ���ų�����______�����ţ�������������Ҫͨ��ʵ�������֤��
��2������ͼ��ʵ�����ṩ����ʢ��ҩƷ����������֤����ֽ�Ŀ��ܲ��
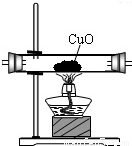 | 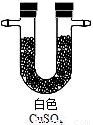 | 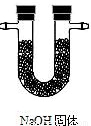 | 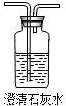 |
| A | B | C | D |
��֤�����к��ж�����̼����Ӧ�Ļ�ѧ����ʽ��______��
�������������������ظ�ʹ�ã���װһ��װ�ã�ͨ��һ��ʵ����֤����ֽ�Ŀ��ܲ��������ͨ�����Ⱥ�˳�����ӵ�����������______����A��B��C��D��ʾ����
�ܴӻ��������Ƕȳ�������ʵ���������β���Ĵ���������______��