��Ŀ����
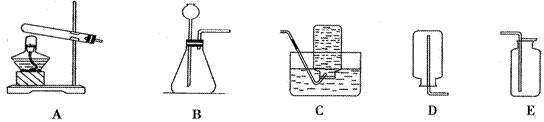
6����ѡ�������װ������ͼ��ʾ��ͼ��A-GΪװ�ô��ţ���-��Ϊ�����Ľӿڷ��ţ�����ѡ���ҩƷ�У������ơ�ͭƬ��п����̿�ۡ����ᡢϡ���ᣮ

����������⣺
��1��д��Bװ���е��������ƣ���
��2��ʵ������ȡ����ʱ����ѡ�õ�ҩƷ��
��3����һ��ѧ��ѡ������Ϊԭ��֮һ�����Ƶõ������лẬ������������ᣬ��������ͨ��װ�ã�дװ�ô��ţ�
��4������ȷ������������ȡ�����������ռ�������װ�ã�д�ӿڷ��ţ�

����������⣺
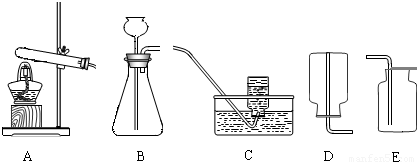
��1��д��Bװ���е��������ƣ���
����̨
������Һ©��
�����Թ�
����2��ʵ������ȡ����ʱ����ѡ�õ�ҩƷ��
�
��ϡ����
��������Ӧ�Ļ�ѧ����ʽΪZn+H2SO4�TZnSO4+H2��
����3����һ��ѧ��ѡ������Ϊԭ��֮һ�����Ƶõ������лẬ������������ᣬ��������ͨ��װ�ã�дװ�ô��ţ�
A
���������ɳ�ȥ����ѧ��Ӧ����ʽΪNaOH+HCl�TNaCl+H2O
����ȡ���ռ���װ���ǣ�дװ�ô��ţ�G
��E
����4������ȷ������������ȡ�����������ռ�������װ�ã�д�ӿڷ��ţ�
��
���٣���ڣ�
���ڣ���٣�
����
����������1����������ͼʾ�������ã���ʶ����������д���������ƣ�
��2��ʵ������ȡ����ѡ��ҩƷ�ķ�Ӧ��ѧ����ʽ�Ŀ��飻
��3��ѡ��ϡ������ȡ����ʱ����������������ϡ����ӷ�����HCl���壬�������ͨ���������ƿɾ���������
���ݷ���װ�õ�װ��ͼʾ������װ��ͼ�е���ȷװ�ã�ѡȡ��ȷװ����ȡ������
��4����������ȡ�����������ռ�������װ�õ�����˳����װ�á�������������ռ�װ�ã���������и����Ա�֤���ռ�������Ϊ��������壬�����������˳��ߵ��������������ûͨ���ٴθ�������ܵõ���������壮
��2��ʵ������ȡ����ѡ��ҩƷ�ķ�Ӧ��ѧ����ʽ�Ŀ��飻
��3��ѡ��ϡ������ȡ����ʱ����������������ϡ����ӷ�����HCl���壬�������ͨ���������ƿɾ���������
���ݷ���װ�õ�װ��ͼʾ������װ��ͼ�е���ȷװ�ã�ѡȡ��ȷװ����ȡ������
��4����������ȡ�����������ռ�������װ�õ�����˳����װ�á�������������ռ�װ�ã���������и����Ա�֤���ռ�������Ϊ��������壬�����������˳��ߵ��������������ûͨ���ٴθ�������ܵõ���������壮
����⣺��1��������Ϊ����װ�õ�����̨����Ϊ���ӷ�Ӧ��Һ�������ķ�Һ©������Ϊ����ҩƷ��Ӧ�������Թܣ�
�ʴ�����̨����Һ©�����Թܣ�
��2��ʵ���ҳ����벻�ӷ���ϡ���ᷴӦ�ٶ����е�п����ȡ������п�����ᷢ���û���Ӧ�ų�������
�ʴ�п����ϡ���ᣬZn+H2SO4�TZnSO4+H2����
��3��װ��A��װ���������ƣ�������������е�HCl���巢���кͷ�Ӧ����ȥ�����ѻ������ͨ��װ��A��ȥ������
��ѡA��NaOH+HCl�TNaCl+H2O��
ʹ�ù���п����Һ��ϡ���ᷴӦ��ȡ������ѡ��Һ�����ȡ��ͷ���װ�ã�����װ��B�ĵ�����������Ǵ���װ�ã����Է���װ��ѡG��
�ܶ�С�ڿ�����Ӧѡ�������ſ����ռ�����ķ�����ͼ��װ��C�Թܿڱ��ܷⲻ�������ռ����壬װ��D�ڵ���û�����Թܵף��ռ����岻���������ռ�����Ӧѡװ��E��
�ʴ�G��E��
��4��������ȷ������˳����װ�á�������������ռ�װ�ã�װ�����ӵĽӿ�˳��Ϊ������١��ڡ��ޣ��ӿڢ٢ڲ����Ⱥ���������˳��Ҳ����Ϊ������ڡ��١��ޣ�
�ʴ𣺢࣬�٣���ڣ����ڣ���٣����ޣ�
�ʴ�����̨����Һ©�����Թܣ�
��2��ʵ���ҳ����벻�ӷ���ϡ���ᷴӦ�ٶ����е�п����ȡ������п�����ᷢ���û���Ӧ�ų�������
�ʴ�п����ϡ���ᣬZn+H2SO4�TZnSO4+H2����
��3��װ��A��װ���������ƣ�������������е�HCl���巢���кͷ�Ӧ����ȥ�����ѻ������ͨ��װ��A��ȥ������
��ѡA��NaOH+HCl�TNaCl+H2O��
ʹ�ù���п����Һ��ϡ���ᷴӦ��ȡ������ѡ��Һ�����ȡ��ͷ���װ�ã�����װ��B�ĵ�����������Ǵ���װ�ã����Է���װ��ѡG��
�ܶ�С�ڿ�����Ӧѡ�������ſ����ռ�����ķ�����ͼ��װ��C�Թܿڱ��ܷⲻ�������ռ����壬װ��D�ڵ���û�����Թܵף��ռ����岻���������ռ�����Ӧѡװ��E��
�ʴ�G��E��
��4��������ȷ������˳����װ�á�������������ռ�װ�ã�װ�����ӵĽӿ�˳��Ϊ������١��ڡ��ޣ��ӿڢ٢ڲ����Ⱥ���������˳��Ҳ����Ϊ������ڡ��١��ޣ�
�ʴ𣺢࣬�٣���ڣ����ڣ���٣����ޣ�
������Ҫ����H2��ʵ�����Ʒ���ԭ��װ�á��ռ������ȣ���װ�õ�����Ҫ�˽�һ���ԭ����װ�á�������������ռ�װ�ã�

��ϰ��ϵ�д�
 �Ͻ�ƽСѧ��������ϵ�д�
�Ͻ�ƽСѧ��������ϵ�д�
�����Ŀ