��Ŀ����
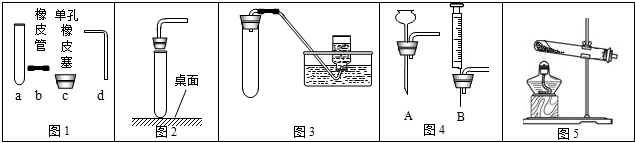
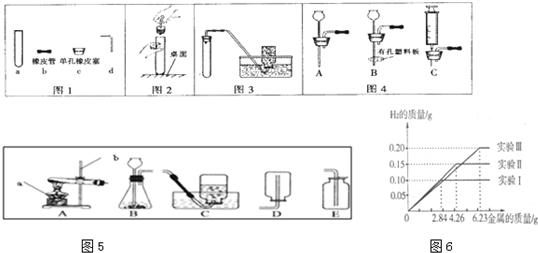
��9�֣���ѧ��һ����ʵ��Ϊ�����Ŀ�ѧ���߱������Ļ�ѧʵ�鼼���ǽ��л�ѧ̽����Ļ����ͱ�֤��ͬʱҲ��ӳ��ʵ���ߵĻ�ѧѧ��������
��һ������ͼ��ʵ���ҳ��õ����������Ʊ����ռ��������Ķ��װ�á�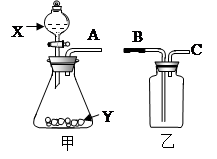
(1)�����������������Լ�X����___ _____����ͼʾ���õ���װ���ռ�������AӦ��________ (�B����C��)������
(2)��X��ϡ���ᡢY��ʯ��ʯ���üס�����װ�ÿ���ɷ������������ռ�CO2�����ϵ��װ�á������з��뱥��NaHCO3��Һ�������Һ�������dz�ȥ____����Ӧ�Ļ�ѧ���̣����� ���������������з���Ũ���ᣬŨ�����������____________________________��
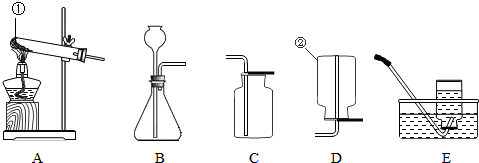
������������ͼװ�ÿ���CO��ԭFe2O3����ʵ�飬������÷�Ӧ���ɵ���������֪��Aװ����ȡ��CO�����л���������CO2��������������⣺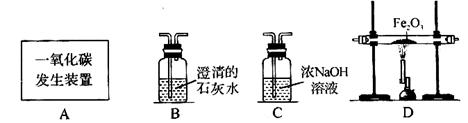
��1������ͨ��װ�õ�˳����A�� (װ�ò����ظ�ʹ��)��
��2��������Dװ����Fe2O3��ĩ�ڷ�Ӧ�����1.6g����Bװ���ڷ�Ӧ������ g��
��3���ӻ����Ƕȿ��ǣ�������װ�õĸĽ���ʩ�� ��
��һ��(1) ����������Һ�� B
(2)�Ȼ������壻NaHCO3 + HCl ="==" NaCl + H2O + CO2�� ����������ȥˮ����
��������1��C��D��B ��2��4.4 ��3����β����ȼ������ռ��ȡ�
����

 ��У����ϵ�д�
��У����ϵ�д�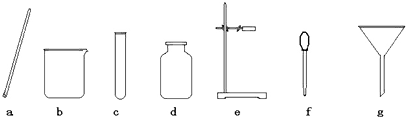
 20����ѧ��һ����ʵ��Ϊ�����Ŀ�ѧ���淶��ʵ�������ʵ��ɹ���ǰ�ᣬ��ش�
20����ѧ��һ����ʵ��Ϊ�����Ŀ�ѧ���淶��ʵ�������ʵ��ɹ���ǰ�ᣬ��ش�