��Ŀ����
����Ŀ����������Na2O2��һ�ֹ���������ˮ��Ӧ�Ļ�ѧ����ʽΪ2Na2O2+2H2O=4NaOH+O2����һ������Na2O2���뵽һ����ˮ�У�Na2O2��ȫ��Ӧ��������Һ������Ϊ100g���ȷ�Ӧǰ����������������3.2g(����ˮ�Ļӷ����Բ���)������㣺
(1)��������������Ϊ________g
(2)��Ӧ��������Һ��NaOH������(д���������)_________
(3)������Ӧ����Һϡ��Ϊ������������Ϊ10%��NaOHҺ����Ҫ��ˮ������Ϊ_________g
���𰸡�3.2 16g 60
��������
��1����ȫ��Ӧ��������Һ�������ͷ�Ӧ���������֮���Ϊ������������������������������Ϊ3.2g��
��2���裺�μӷ�Ӧ�Ĺ������Ƶ�����Ϊx�������������Ƶ�����Ϊy��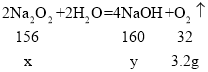
![]() x=15.6g��
x=15.6g��
![]() y=16g��
y=16g��
�𣺷�Ӧ��������Һ�����ʵ�����Ϊ16g��
��3���裺��Ҫ����ˮ������Ϊz��
![]() z=60g��
z=60g��
����Ҫ����ˮ������Ϊ60g��

 ������ϰ�ο����뵥Ԫ���ϵ�д�
������ϰ�ο����뵥Ԫ���ϵ�д�����Ŀ����ͼ��ʾ���ڷֱ�ʢ�� 100g ˮ���ձ��з��� KNO3 ����ܽ⣬��˵����ȷ����
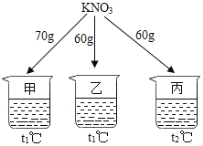
�¶ȣ����� | t1 | t2 |
KNO3���ܽ�ȣ�g/100g ˮ�� | 60 | 90 |
A. �ס��һ�Ϻ�Ϊt1��IJ�������Һ
B. ��������t2�棬��Һ��������
C. �ҡ�����Һ�������������
D. ��������t1�棬�о�������