��Ŀ����
 10����������������ѧ��Ӧ����ʽ�ش����⣺
10����������������ѧ��Ӧ����ʽ�ش����⣺A��2KOH+SO3��K2SO4+H2O��B��2HClO��2HCl+O2�� C��H2CO3��H2O+CO2��
��1�������о���ѧϰ��ѧ��һ�ֳ��õķ���������������ѧ����ʽ���漰�������������ʣ��������ڵ��ʵ���
02
��������������ǣ�ֻҪд������һ�֣�H2O
���������K2SO4
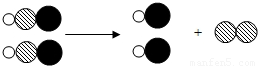
����2����ͼ��ij��ѧ��Ӧ��ʾ��ͼ������
 ��
�� ��
�� �ֱ��ʾ����ԭ�ӣ�����������Ӧ�������������
�ֱ��ʾ����ԭ�ӣ�����������Ӧ�������������B
�����ţ�����3����A��B������Ӧ�漰����������ѡ���ʵ������ʣ�д��һ�������кͷ�Ӧ�Ļ�ѧ����ʽ
KOH+HCl�TKCl+H2O����KOH+HClO�TKClO+H2O��
�����������Ը������ʵ�������ɼ���Ԫ����ɿ����ж����ʵ���𣬸��ݷ�Ӧ��������P�������غ㶨�ɿ�����д��ѧ����ʽ����ͼӦ�����κ�ˮ�ķ�Ӧ���кͷ�Ӧ��
����⣺��1����������ͬ��Ԫ����ɵĴ�������ڵ��ʣ�ˮ������Ԫ�غ���Ԫ����ɵĻ���������������������ɽ������Ӻ����������ɵĻ���������Σ����02��H2O��K2SO4
��2����ͼ�п�֪��B�Ļ�ѧ����ʽ��ͼ����Ϣ���ϣ����B
��3�������кͷ�Ӧ�Ļ�ѧ��Ӧ�У�KOH+HCl�TKCl+H2O����KOH+HClO�TKClO+H2O����
��2����ͼ�п�֪��B�Ļ�ѧ����ʽ��ͼ����Ϣ���ϣ����B
��3�������кͷ�Ӧ�Ļ�ѧ��Ӧ�У�KOH+HCl�TKCl+H2O����KOH+HClO�TKClO+H2O����
������������Ҫ�����˻�ѧ����ʽ����д�������ʵķ�����ȷ�������ݣ�

��ϰ��ϵ�д�
�����Ŀ
 ���͡�
���͡� ���ֱ��ʾ����ԭ�ӣ�
���ֱ��ʾ����ԭ�ӣ�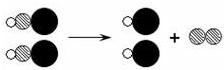

 �ֱ��ʾ����ԭ�ӣ�����������Ӧ�������������
�ֱ��ʾ����ԭ�ӣ�����������Ӧ������������� ������
������ ���͡�
���͡� ���ֱ��ʾ����ԭ�ӣ�
���ֱ��ʾ����ԭ�ӣ�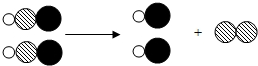
 ������
������ ���͡�
���͡� ���ֱ��ʾ����ԭ�ӣ�
���ֱ��ʾ����ԭ�ӣ�