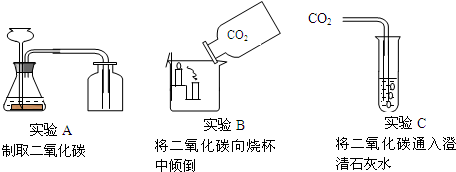
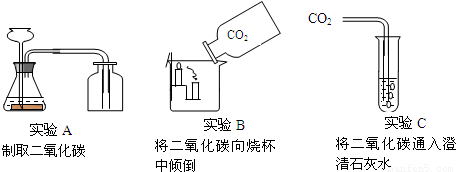
��Ŀ����
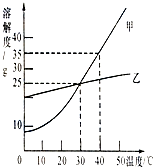 ��ͼ��ʾ����գ�
��ͼ��ʾ����գ���1��40��ʱ���������ܽ��Ϊ
��2���ܽ�����¶ȱ仯Ӱ��Ƚϴ��������
��3��30��ʱ����100gˮ�м���30g�����ʣ�����ܽ��������Һ��
��4����֪��ˮ��Һ�У��Ҵ���C2H5OH�����������룬�⻯�⣨HI��������������ӣ�H+���͵����ӣ�I-�����������Ƶ�����������Ӻ����������ӣ���ش��������⣺
a��������������Һ������ƶϣ�ҽ�þƾ������Ҵ�75%��ˮ��Һ����
b��д����������֮�䷢���кͷ�Ӧ�Ļ�ѧ����ʽ
c������������Һ�д��ڵ������У��û�ѧ���ű�ʾ��
��������1�����ܽ�����߿�֪ij�¶������� ���ܽ�ȣ�
��2�����ܽ�����߿�֪���ʵ��ܽ�����¶�Ӱ�������
��3���ݸ��¶������ʵ��ܽ�ȡ������ʵ����������������
��4��a��������ˮ��Һ�У��Ҵ���C2H5OH�����������룬�⻯�⣨HI����������������Ӷ��������ӣ�H+������������з������
b���кͷ�Ӧ����ͼ���ķ�Ӧ���ݴ���д����ʽ��
c���������Ƶ�����������Ӻ����������ӣ��ܼ�ˮ��ˮ���ӹ��ɣ�
��2�����ܽ�����߿�֪���ʵ��ܽ�����¶�Ӱ�������
��3���ݸ��¶������ʵ��ܽ�ȡ������ʵ����������������
��4��a��������ˮ��Һ�У��Ҵ���C2H5OH�����������룬�⻯�⣨HI����������������Ӷ��������ӣ�H+������������з������
b���кͷ�Ӧ����ͼ���ķ�Ӧ���ݴ���д����ʽ��
c���������Ƶ�����������Ӻ����������ӣ��ܼ�ˮ��ˮ���ӹ��ɣ�
����⣺��1��40��ʱ���������ܽ��Ϊ35g��
��2����ͼ��֪�����ܽ�����¶ȱ仯Ӱ��Ƚϴ�
��3��30��ʱ�ҵ��ܽ����25g��������100gˮ�м���30g�����ʣ�����ܽ������ܽ�25g��������Һ�DZ�����Һ��������Һ�����ʵ���������Ϊ
��100%=20%��
��4��a�����ھƾ���ˮ��Һ�в��������룬��������Һ��Ҳ��û�������ӻ��������������ӣ����Ծƾ���Һ�Ȳ�������Ҳ���Լ��ԣ����⻯���ܷ���������������ӣ��������ˮ��Һ�����ԣ�
b�����ڵ⻯�����ʱ���ɵ�������ֻ�������ӣ�˵�������ᣬ�����������Ǽ�����Ƿ����ķ�Ӧ���кͷ�Ӧ����Ӧ����ʽ�ǣ�HI+NaOH=NaI+H2O��
c������������Һ���������Ƶ�ˮ��Һ�����������ӡ������ӣ�ˮ���ӣ���д���ӷ���ʱ��д���֡��ٱ�������ΪH2O��Na+��OH-
�ʴ�Ϊ����1��35����2���ף���3�����ͣ�20%����4��a�����ԣ� ���ԣ�b��HI+NaOH=NaI+H2O��c��Na+��Cl-��H2O��
��2����ͼ��֪�����ܽ�����¶ȱ仯Ӱ��Ƚϴ�
��3��30��ʱ�ҵ��ܽ����25g��������100gˮ�м���30g�����ʣ�����ܽ������ܽ�25g��������Һ�DZ�����Һ��������Һ�����ʵ���������Ϊ
| 25g |
| 125g |
��4��a�����ھƾ���ˮ��Һ�в��������룬��������Һ��Ҳ��û�������ӻ��������������ӣ����Ծƾ���Һ�Ȳ�������Ҳ���Լ��ԣ����⻯���ܷ���������������ӣ��������ˮ��Һ�����ԣ�
b�����ڵ⻯�����ʱ���ɵ�������ֻ�������ӣ�˵�������ᣬ�����������Ǽ�����Ƿ����ķ�Ӧ���кͷ�Ӧ����Ӧ����ʽ�ǣ�HI+NaOH=NaI+H2O��
c������������Һ���������Ƶ�ˮ��Һ�����������ӡ������ӣ�ˮ���ӣ���д���ӷ���ʱ��д���֡��ٱ�������ΪH2O��Na+��OH-
�ʴ�Ϊ����1��35����2���ף���3�����ͣ�20%����4��a�����ԣ� ���ԣ�b��HI+NaOH=NaI+H2O��c��Na+��Cl-��H2O��
��������ȷ�ܽ�ȸ���ܽ�����ߵ����塢�������������ļ��㷽�����кͷ�Ӧ��֪ʶ������֪ʶǨ��ת����Ӧ��������������ѧ�������������ͷ���������

��ϰ��ϵ�д�
�����Ŀ
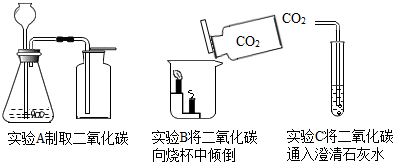
 ���Ѿ��˽ⳣ��������ʹ�÷�����������ͼ��ʾ������գ�
���Ѿ��˽ⳣ��������ʹ�÷�����������ͼ��ʾ������գ�