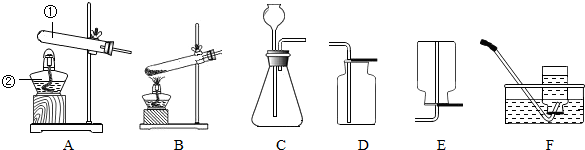
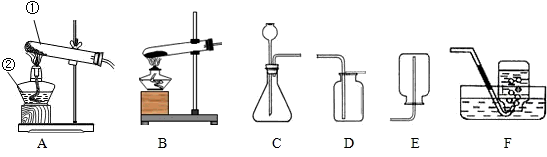
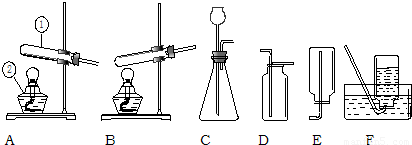
��Ŀ����
22����������װ����ȡ���г��������壮��ش�
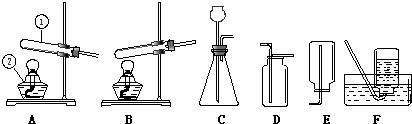
��1��д�������١��ڵ����ƣ�����
��2���ø������������ʱ��Ӧѡ�õķ���װ��
��3������Cװ�ã�ͨ���ֽⷴӦ���Ƶõ������ǣ�д��ѧʽ��
��4������Cװ�ã�ͨ�����ֽⷴӦ���Ƶõ������ǣ�д��ѧʽ��
��5������װ��A�����Եľ��巽����

��1��д�������١��ڵ����ƣ�����
�Թ�
�������ƾ���
����2���ø������������ʱ��Ӧѡ�õķ���װ��
A
���÷�Ӧ�Ļ�ѧ����ʽΪ2KMnO4=K2MnO4+MnO2+O2��
����3������Cװ�ã�ͨ���ֽⷴӦ���Ƶõ������ǣ�д��ѧʽ��
O2
���÷�Ӧ�Ļ�ѧ����ʽΪ2H2O2�T2H2O+O2��
�����ռ�װ��Ӧѡ��D��F
�����ţ�����4������Cװ�ã�ͨ�����ֽⷴӦ���Ƶõ������ǣ�д��ѧʽ��
CO2
�����ռ�װ��Ӧѡ��D
�����ţ�����5������װ��A�����Եľ��巽����
���䵼�ܿ�һ�˽���ˮ�У������ֽ���ס�Թܣ����ˮ�в����������ݣ�˵������������
�����������������ڻ�����ʵ�鼼��ר�⣬�ڿ������������ͬʱ�ּ�����һ������װ�ã�ֻҪ��ȷ�������������Ҫ���ȵ�װ�ã�B�е��Թܿڳ����Ǹ���������������л�Ҫ��ȷ������ķ�Ӧ�ķ���ʽ������һ��������Ӧ���ͣ�ǰ���Ӧ�Ϳ��Խ�����⣻�ռ�װ�ø���������������ʾͿ���ѡ��
����⣺��1�����ʵ�����������Ƽ��ɣ��ʴ�Ϊ���Թ� �ƾ��ƣ�2�֣�
��2���ø��������������Ҫ���ȣ�B�Ǹ�����װ�ã���ѡA��1�֣�2KMnO4=K2MnO4+MnO2+O2����2�֣�
��3��Cװ�ò���Ҫ���ȣ�����Ҫ�÷ֽⷴӦ�����壬��Ӧ�����������������ռ����������֣��ʴ�Ϊ��O2��1�֣�2H2O2�T2H2O+O2����2�֣�D��F��1�֣�
��4��Cװ�ò���Ҫ���ȣ�ͨ�����ֽⷴӦ���Ƶõ������Ƕ�����̼����ѡCO2��1�֣�D��1�֣�
��5��������װ�õ����������ʵ������������ʴ�Ϊ�����䵼�ܿ�һ�˽���ˮ�У�1�֣��������ֽ���ס�Թܣ�0.5�֣������ˮ�в����������ݣ�0.5�֣���˵�����������ã�
��2���ø��������������Ҫ���ȣ�B�Ǹ�����װ�ã���ѡA��1�֣�2KMnO4=K2MnO4+MnO2+O2����2�֣�
��3��Cװ�ò���Ҫ���ȣ�����Ҫ�÷ֽⷴӦ�����壬��Ӧ�����������������ռ����������֣��ʴ�Ϊ��O2��1�֣�2H2O2�T2H2O+O2����2�֣�D��F��1�֣�
��4��Cװ�ò���Ҫ���ȣ�ͨ�����ֽⷴӦ���Ƶõ������Ƕ�����̼����ѡCO2��1�֣�D��1�֣�
��5��������װ�õ����������ʵ������������ʴ�Ϊ�����䵼�ܿ�һ�˽���ˮ�У�1�֣��������ֽ���ס�Թܣ�0.5�֣������ˮ�в����������ݣ�0.5�֣���˵�����������ã�
�����������������ǿ����װ��ͼ�м���һ�����������������ѧ����������������ͬʱ��Ҳ�ܴ���ѧ���Ӳ�ͬ�Ƕ�˼�����⣮

��ϰ��ϵ�д�
�����Ŀ