��Ŀ����
 δ�����˺�����������Ҫ���Ƶ���Ҫ��Ⱥ��Ŀǰ�����������ಹ�Ƶĸ�Ƭ���ۣ���ͼ�Ǹ��иǸ߸�Ƭ�IJ���˵����ij������ȤС��������˵������α�����������µ�ʵ�飺
δ�����˺�����������Ҫ���Ƶ���Ҫ��Ⱥ��Ŀǰ�����������ಹ�Ƶĸ�Ƭ���ۣ���ͼ�Ǹ��иǸ߸�Ƭ�IJ���˵����ij������ȤС��������˵������α�����������µ�ʵ�飺��ȡ1ƬƬ������
�ڽ����������ϡ������
����ȫ��Ӧ���ռ���0.55g������̼
�Է�����
��1���߸�Ƭ��̼��Ƶ�����������
��2��ͨ�������жϴ˸�Ƭ�иƺ������ע�Ƿ���ʵ�����ٶ���Ƭ�������ɷݲ������ᷴӦ��1g=1000mg��
���㣺���ݻ�ѧ��Ӧ����ʽ�ļ���,��������ijԪ�ص���������
ר�⣺�йػ�ѧ����ʽ�ļ���
��������1������̼��������ᷴӦ�ķ���ʽ����������̼���������������ж�̼��Ƶ�������
��2��̼��Ƶ��������Ը���̼�������ռ�е����������������Ԫ�ص������벹�Ƽ���˵���Ƚ��ж��Ƿ���ʵ��
��2��̼��Ƶ��������Ը���̼�������ռ�е����������������Ԫ�ص������벹�Ƽ���˵���Ƚ��ж��Ƿ���ʵ��
����⣺
��1����������������̼�����ȫ��Ӧ��
���Ƭ��̼��Ƶ�����Ϊx
CaCO3+2HCl�TCaCl2+CO2��+H2O
100 44
x 0.55g
=
x�T1.25g
�߸�Ƭ��̼��Ƶ���������=
��100%=50%
��2����1.25g̼����к���Ԫ�ص�����Ϊ��1.25g��
��100%=0.5g
0.5g��1000mg/g=500mg
��˵����ÿƬ����500mg��һ�£���˵����ʵ��
�𰸣�
��1���߸�Ƭ��̼��Ƶ���������50%
��2���˸�Ƭ�иƺ������ע��ʵ��
��1����������������̼�����ȫ��Ӧ��
���Ƭ��̼��Ƶ�����Ϊx
CaCO3+2HCl�TCaCl2+CO2��+H2O
100 44
x 0.55g
| 100 |
| 44 |
| x |
| 0.55g |
x�T1.25g
�߸�Ƭ��̼��Ƶ���������=
| 1.25g |
| 2.5g |
��2����1.25g̼����к���Ԫ�ص�����Ϊ��1.25g��
| 40 |
| 40+12+16��3 |
0.5g��1000mg/g=500mg
��˵����ÿƬ����500mg��һ�£���˵����ʵ��
�𰸣�
��1���߸�Ƭ��̼��Ƶ���������50%
��2���˸�Ƭ�иƺ������ע��ʵ��
���������⿼�����û�ѧʽ�ͻ�ѧ��Ӧ����ʽ�ļ��㣬ѧ����ȷ����ѧʽ����ķ�������ѧ��Ӧ�к������ʵ������ɴ��뷽��ʽ�����ǽ��Ĺؼ���

��ϰ��ϵ�д�
 �����ܿ����ϵ�д�
�����ܿ����ϵ�д�
�����Ŀ
�����������ڴ�������ǣ�������
| A������ʯ | B��ϡ���� |
| C��������̼ | D��ʯ��ˮ |


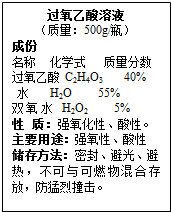 ij���۹������ᣨ��ѧʽΪ��C2H4O3����Һ�����ǩ�ϵIJ�������˵����ͼ��
ij���۹������ᣨ��ѧʽΪ��C2H4O3����Һ�����ǩ�ϵIJ�������˵����ͼ��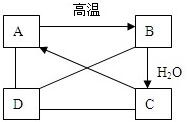 ��A��B��C��D�������ʣ���ͼ��ʾ��A��B��C��һ�������¿��Է���ת������C��Һ��ͨ��CO2����Һ����ǣ����ɰ�ɫ����A��D��A��B��C���ܷ�����Ӧ��D��C�����кͷ�Ӧ��D��A��Ӧ��CO2���������D��AgNO3��Һ��Ӧ���ɲ���������ϡ����İ�ɫ������
��A��B��C��D�������ʣ���ͼ��ʾ��A��B��C��һ�������¿��Է���ת������C��Һ��ͨ��CO2����Һ����ǣ����ɰ�ɫ����A��D��A��B��C���ܷ�����Ӧ��D��C�����кͷ�Ӧ��D��A��Ӧ��CO2���������D��AgNO3��Һ��Ӧ���ɲ���������ϡ����İ�ɫ������