��Ŀ����
��2013?������һģ������һ����������������������Һ���йز�����ͼ��ʾ��
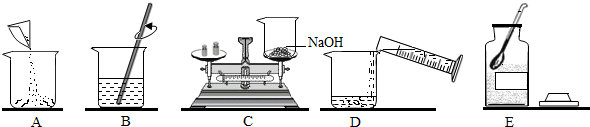
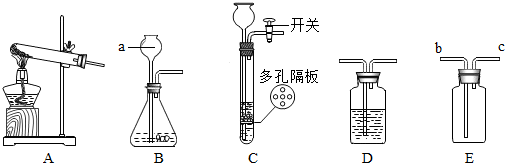
��1������ʵ�����ȷ����˳��Ϊ
��2����pH��ֽ�ⶨ��������Һ�����ȣ���������Ϊ
��3������һ��Ʒ�����������������ء�̼��غ�ˮ�������ʣ������Ʒ�м���������������Ϊ9.8%��ϡ����100g��ǡ����ȫ��Ӧ�õ�������Һ���Լ��㣺���ɸ���Һ�ɵõ��Ĺ��������

��1������ʵ�����ȷ����˳��Ϊ
ECADB
ECADB
������ĸ���ű�ʾ����C�����г��ֵĴ������뵹�ã�����˵�����ɣ�
���뵹�ã�����˵�����ɣ�
����C�����������Ϊ15g������Ķ���Ϊ3.5g�����������ƺ��ձ���ʵ��������Ϊ11.5
11.5
g����2����pH��ֽ�ⶨ��������Һ�����ȣ���������Ϊ
�ø���IJ�����պȡ����ͷ�ι���ȡ���ٴ�����Һ�����ڷ��ڸ���IJ������״ɰ��ϵĸ���pH��ֽ�ϣ��ٰ���ֽ��ʾ����ɫ�����ɫ���Ƚϣ����ɵó�������Һ��pH
�ø���IJ�����պȡ����ͷ�ι���ȡ���ٴ�����Һ�����ڷ��ڸ���IJ������״ɰ��ϵĸ���pH��ֽ�ϣ��ٰ���ֽ��ʾ����ɫ�����ɫ���Ƚϣ����ɵó�������Һ��pH
����3������һ��Ʒ�����������������ء�̼��غ�ˮ�������ʣ������Ʒ�м���������������Ϊ9.8%��ϡ����100g��ǡ����ȫ��Ӧ�õ�������Һ���Լ��㣺���ɸ���Һ�ɵõ��Ĺ��������
17.4g
17.4g
����������1��������Һ���ƵIJ��衢ע���������������뵹��ʱ��ʵ�ʳ����������=���������-�����������
��2��������pH��ֽ�ⶨ��ҺpHֵ�ķ����ش�
��3���������ء�̼��������ᷴӦ����������أ����������غ㶨��������е���������������ᣬ��������������غ㼰��ϵʽ�����н��
��2��������pH��ֽ�ⶨ��ҺpHֵ�ķ����ش�
��3���������ء�̼��������ᷴӦ����������أ����������غ㶨��������е���������������ᣬ��������������غ㼰��ϵʽ�����н��
����⣺��1��������Һ��һ�㲽���ǣ����㡢�������ܽ⡢װƿ����ʵ�����ȷ˳���ǣ�ȡҩƷ�����������ձ�����ȡˮ�������ձ��������ܽ⣻C�����г��ֵĴ����ǽ����뵹�ã�����ƽ����ʱ��������Ӧ�������̣�����������̣��������ƺ��ձ���ʵ��������Ϊ15g-3.5g=11.5g��
��2����pH��ֽ�ⶨ��������Һ�����ȣ��ⶨ�������ø���IJ�����պȡ����ͷ�ι���ȡ�������Ĵ�����Һ�������ڷ��ڸ���IJ������״ɰ��ϵĸ���pH��ֽ�ϣ��ٰ���ֽ��ʾ����ɫ�����ɫ���Ƚϣ����ɵó�������Һ��pH��
��5����Ӧ����Һ�����ԣ�˵�������е������ǡ��ȫ��ת��Ϊ����أ�
����������غ㼰����֮��Ĺ�ϵʽ��֪��H2SO4��K2SO4��֪��
���õ��Ĺ�������ص�����Ϊ��100g��9.8%��
=17.4g��
�ʴ�Ϊ����1��ECADB�����뵹�ã�11.5g��
��2���ø���IJ�����պȡ����ͷ�ι���ȡ���ٴ�����Һ�����ڷ��ڸ���IJ������״ɰ��ϵĸ���pH��ֽ�ϣ��ٰ���ֽ��ʾ����ɫ�����ɫ���Ƚϣ����ɵó�������Һ��pH��
��3��17.4g��
��2����pH��ֽ�ⶨ��������Һ�����ȣ��ⶨ�������ø���IJ�����պȡ����ͷ�ι���ȡ�������Ĵ�����Һ�������ڷ��ڸ���IJ������״ɰ��ϵĸ���pH��ֽ�ϣ��ٰ���ֽ��ʾ����ɫ�����ɫ���Ƚϣ����ɵó�������Һ��pH��
��5����Ӧ����Һ�����ԣ�˵�������е������ǡ��ȫ��ת��Ϊ����أ�
����������غ㼰����֮��Ĺ�ϵʽ��֪��H2SO4��K2SO4��֪��
���õ��Ĺ�������ص�����Ϊ��100g��9.8%��
| 174 |
| 98 |
�ʴ�Ϊ����1��ECADB�����뵹�ã�11.5g��
��2���ø���IJ�����պȡ����ͷ�ι���ȡ���ٴ�����Һ�����ڷ��ڸ���IJ������״ɰ��ϵĸ���pH��ֽ�ϣ��ٰ���ֽ��ʾ����ɫ�����ɫ���Ƚϣ����ɵó�������Һ��pH��
��3��17.4g��
���������⿼��֪ʶ�ۺϣ�������Һ�����ơ��ⶨ��Һ�����ȡ������غ㶨�ɵ�Ӧ�õ�֪ʶ�ǽ�����Ļ����ؼ���Ҫ������������ϸ����һ�������

��ϰ��ϵ�д�
 ��������ϵ�д�
��������ϵ�д�
�����Ŀ
��2013?������һģ���±���KCl�IJ����ܽ�����ݣ�����˵����ȷ���ǣ�������
|