��Ŀ����
(6��)ͭ�������������õĽ���֮һ��
��1������ͭ��Ʒ�У����ý��������Ե��� (����ĸ���)��

A��ͭ�ʽ��� B��ͭ���� C��ͭ���
��2����֤ͭ�����Ļ�ԣ�����ѡ�õ��Լ��� ��
��3�����÷Ͼɵ��ͭñ(��Cu��Zn)��ȡ����ͭ(Cu)�����õ�����п��Һ����Ҫ�� ������(��Ӧ��������ȥ): ��֪:2Cu+2H2SO4+O2��2CuSO4+2H2O

�� ���̢��з�������������� ��
�� ���̢����������������Ӧ�Ļ�ѧ����ʽΪ ��
��ҺA��һ�����е����ʵ������� ��
��1������ͭ��Ʒ�У����ý��������Ե��� (����ĸ���)��

A��ͭ�ʽ��� B��ͭ���� C��ͭ���
��2����֤ͭ�����Ļ�ԣ�����ѡ�õ��Լ��� ��
��3�����÷Ͼɵ��ͭñ(��Cu��Zn)��ȡ����ͭ(Cu)�����õ�����п��Һ����Ҫ�� ������(��Ӧ��������ȥ): ��֪:2Cu+2H2SO4+O2��2CuSO4+2H2O

�� ���̢��з�������������� ��
�� ���̢����������������Ӧ�Ļ�ѧ����ʽΪ ��
��ҺA��һ�����е����ʵ������� ��
��1��B ��2����������Һ
��3�� �� ���� �� Zn+H2SO4=ZnSO4+H2�� �� ZnSO4 CuSO4
��3�� �� ���� �� Zn+H2SO4=ZnSO4+H2�� �� ZnSO4 CuSO4
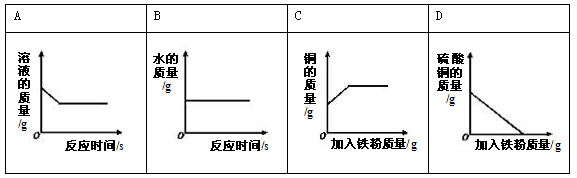
�����������1��A���ƽ���Ʒ���������Ľ�������B��ͭ�������ý��������ԣ�C��ͭ��������õ����ԣ���ѡB
��2����֤�����Ļ�Կ�����2��ԭ����1���������ᷴӦ��2������������Һ��Ӧ��������֤ͭ�����Ļ�ԣ�����ѡ�õ��Լ��ǣ�Cu����������Һ
��3���ٹ��̢��н��з�������ֱ�õ��������Һ�����������ǣ����ˣ�
�����ڷϾɵ��ͭñ���� Cu��Zn���ڼ���ͬʱϡ����������������ṩ����Ϣ������ͭ��п���ᷢ����Ӧ������ҺAӦ������п������ͭ�Ļ����Һ����Ҫ�õ�����п��Һ������BӦ��ΪZn�����Թ��̢����������������Ӧ�Ļ�ѧ����ʽΪ��Zn+H2SO4=ZnSO4+H2��
����ҺA��һ�����е����ʵ������У�ZnSO4��CuSO4

��ϰ��ϵ�д�
�����Ŀ