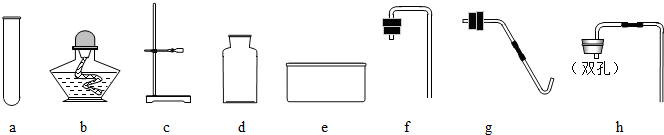
��Ŀ����
24��ʵ�����ü��ȸ������������������ˮ���ռ����ش��������⣺
��1����ȡ����ǰҪ�ȼ���װ�������ԣ�Ŀ���Ƿ�ֹװ��
��2�������Թܿ���һ��������Ŀ����
��3�������������Թ���ĵ��ܲ��˹�����ԭ����
��4���Թܿ���������б����ֹ
��5������ʱ�Ƚ��ƾ������Թ��·������ƶ�����Ŀ����
��1����ȡ����ǰҪ�ȼ���װ�������ԣ�Ŀ���Ƿ�ֹװ��
©��
����2�������Թܿ���һ��������Ŀ����
��ֹ������ط�ĩ���ȷɽ����뵼�ܣ���������
����3�������������Թ���ĵ��ܲ��˹�����ԭ����
�ž�ƿ�ڵĿ������õ��ϴ�������
����4���Թܿ���������б����ֹ
����ˮ����ʹ�Թ�ը��
����5������ʱ�Ƚ��ƾ������Թ��·������ƶ�����Ŀ����
���Թܾ�������
����������ȷ����ʵ���в�����Ŀ�ģ���ʵ���������֪��Ȼ֪������Ȼ����˳�����������ȡ�ı��ϣ�
����⣺��1����ȡ����ǰҪ�ȼ���װ�������ԣ�Ŀ���Ƿ�ֹװ�� ©����
�ʴ�Ϊ��©����
��2�������Թܿ���һ��������Ŀ���ǣ���ֹ������ط�ĩ���ȷɽ����뵼�ܣ��������ܣ�
�ʴ�Ϊ����ֹ������ط�ĩ���ȷɽ����뵼�ܣ��������ܣ�
��3�������������Թ���ĵ��ܹ�����������Թ��ڵĿ���������ȫ�ų���������ȡ�������У�
�ʴ�Ϊ���ž�ƿ�ڵĿ������õ��ϴ������壻
��4���Թܿ���������б����ֹ������ˮ����ʹ�Թ�ը�ѣ�
�ʴ�Ϊ������ˮ����ʹ�Թ�ը�ѣ�
��5������ʱ�Ƚ��ƾ������Թ��·������ƶ�����Ŀ���ǣ����Թܾ������ȣ�
�ʴ�Ϊ�����Թܾ������ȣ�
�ʴ�Ϊ��©����
��2�������Թܿ���һ��������Ŀ���ǣ���ֹ������ط�ĩ���ȷɽ����뵼�ܣ��������ܣ�
�ʴ�Ϊ����ֹ������ط�ĩ���ȷɽ����뵼�ܣ��������ܣ�
��3�������������Թ���ĵ��ܹ�����������Թ��ڵĿ���������ȫ�ų���������ȡ�������У�
�ʴ�Ϊ���ž�ƿ�ڵĿ������õ��ϴ������壻
��4���Թܿ���������б����ֹ������ˮ����ʹ�Թ�ը�ѣ�
�ʴ�Ϊ������ˮ����ʹ�Թ�ը�ѣ�
��5������ʱ�Ƚ��ƾ������Թ��·������ƶ�����Ŀ���ǣ����Թܾ������ȣ�
�ʴ�Ϊ�����Թܾ������ȣ�
������������������ʹ������ȡʱ�ռ�����������ռ������岻������������������

��ϰ��ϵ�д�
�����Ŀ