��Ŀ����
����Ŀ���ռ���нϺõ�ɱ�����������������ã�����ҵ�ռ��г���������̼���ơ�ij��ѧѧϰС��ͬѧΧ�ƹ�ҵ�ռ���Ʒ���Ȳⶨ���⣬չ����������̽����
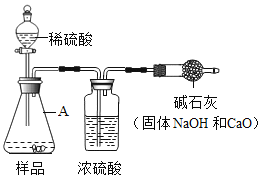
��ԭ��˼·������Na2CO3��ϡH2SO4��Ӧ����CO2��ͨ��CO2 �����IJⶨ��ȷ����Ʒ��̼���Ƶ��������Ӷ�������Ʒ���ȡ�
��ʵ�鷽����С��ͬѧ��������˼·���������ͼ��ʵ�鷽�����̶�װ��ʡ�ԣ�����μ���Aװ�õ�������________________________��
���������ۣ�С����Ϊ��ͼʵ�鷽���������ȱ�ݣ��ᵼ�����Ա����������Ϊ������Щ���ػᵼ��ʵ����ƫ��_____________������ţ���
�������ϡ���������� ��װ���ڿ����е�CO2û���ų�
����Ӧ������CO2δ����ȫ���� ������������ֱ����ͨ
�������Ľ���Ϊ������ʹCO2�����IJⶨ��ȷ����С��ͬѧ�����������ۣ�����ͼ�������иĽ������������ͼ��ʵ�鷽�����̶�װ��ʡ�ԣ���
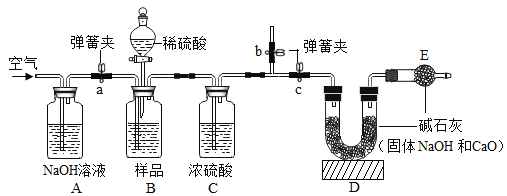
��1��װ��B����Ʒ����ϡ���ᷴӦǰ��ֹͣ��Ӧ��Ҫͨ�����Ŀ�������Ӧǰͨ����ʱa��b��c�������ɼеĿ��Ʒ�����_______________��ֹͣ��Ӧ��ͨ�����Ŀ�����Ŀ����__________��
��2��д��װ��A�з�����Ӧ�Ļ�ѧ����ʽ��_______________________��
�����ݷ�����
��3������Ӧ�����У�װ��B�еμ�ϡ������ٶ�̫�죬���ռ�Ĵ��Ƚ�____________������ƫ��������ƫ����������Ӱ��������
��4����ԭ��Ʒ��Ag��ʵ��������Cװ������Bg��Dװ������Cg����ԭ��Ʒ�Ĵ��ȣ�����������Ϊ_____________________________________��
����չ���죩
��5��Dװ����װ���������ƹ����U�ιܣ�������װ�г���ʯ��ˮ��ϴ��ƿ��ԭ��_________����д��CO2�����ʯ��ˮ��Ӧ�Ļ�ѧ����ʽ��_______________________��
��6��ʵ�������С����С��һ��̽��ʱ�����ְ�Ŀǰ��ѧ֪ʶ��������ʵ�鷽���ⶨ��ҵ�ռ���Ʒ���ȣ�������������һ���·�����____________________________��
���𰸡��رշ�Һ©���Ļ�����Ȼ���ܷ���ˮ�У���˫�ֽ�����ƿ����ڣ��۲쵼�ܿ��Ƿ�������ð�� �٢� �ر�c����a��b ʹ���ɵĶ�����̼������������ȫ���� 2NaOH+CO2=Na2CO3+H2O ƫ�� ![]() ������������ˮ������ʯ��ˮ�����������ɵĶ�����̼��ȫ���� CO2+Ca��OH��2=CaCO3��+H2O ȡһ�������ռ���Ʒ��ȫ�ܽ���ˮ�У�Ȼ������Һ����μ����Ȼ�����Һ��ֱ�����ٲ�������Ϊֹ�����ˡ�ϴ�ӡ��������������������Ȼ���������ɵ�̼�ᱵ���������������û�ѧ����ʽNa2CO3+BaCl2=BaCO3��+2NaCl�����̼���Ƶ��������������ռ���Ʒ���������ɼ�����䴿��.
������������ˮ������ʯ��ˮ�����������ɵĶ�����̼��ȫ���� CO2+Ca��OH��2=CaCO3��+H2O ȡһ�������ռ���Ʒ��ȫ�ܽ���ˮ�У�Ȼ������Һ����μ����Ȼ�����Һ��ֱ�����ٲ�������Ϊֹ�����ˡ�ϴ�ӡ��������������������Ȼ���������ɵ�̼�ᱵ���������������û�ѧ����ʽNa2CO3+BaCl2=BaCO3��+2NaCl�����̼���Ƶ��������������ռ���Ʒ���������ɼ�����䴿��.
��������
[ʵ�鷽��]���װ��A�����Եķ����ǹرշ�Һ©���Ļ�����Ȼ���ܷ���ˮ�У���˫�ֽ�����ƿ����ڣ��۲쵼�ܿ��Ƿ�������ð��������رշ�Һ©���Ļ�����Ȼ���ܷ���ˮ�У���˫�ֽ�����ƿ����ڣ��۲쵼�ܿ��Ƿ�������ð����
[��������]�ټ����ϡ���������㡢��װ���ڿ����е�CO2û���ų����۷�Ӧ������CO2δ����ȫ��������������ᵼ�¶�����̼����ƫ�ͣ��������̼��������ƫС������ɼ�������ռ�Ĵ��Ƚ��ƫ�ߣ����ܸ���������ֱ����ͨ���������ƻ������տ����еĶ�����̼��ˮ��������ɼ������̼���Ƶ�����ƫ���¼�������ռ�Ƚ��ƫ�ͣ�����٢ڢۣ�
[�����Ľ�]��1����Ӧǰͨ����ʱ���ر�c����a��b������B��Cװ���еĶ�����̼�ų���ʹʵ������ȷ��ֹͣ��Ӧ��ͨ�����Ŀ�����Ŀ����ʹ���ɵĶ�����̼������������ȫ���գ�����ر�c����a��b��ʹ���ɵĶ�����̼������������ȫ���գ�
��2������ͨ������������Һʱ�������еĶ�����̼���������Ʒ�Ӧ����̼���ƺ�ˮ�����2NaOH+CO2=Na2CO3+H2O��
[���ݷ���]
��3������Ӧ�����У�װ��B�еμ�ϡ������ٶ�̫�죬�����ɵĶ�����̼�Ͳ��ܱ��������ƹ�����ȫ���գ���ɼ������̼���Ƶ�����ƫС����ô���ռ�Ĵ��Ƚ�ƫ�ߣ����ƫ�ߣ�
��4��Dװ������Cg����������Cg�Ķ�����̼������Ҫ̼���Ƶ�����Ϊx����
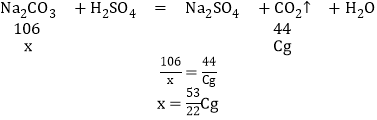
��ԭ��Ʒ�Ĵ��ȣ�����������Ϊ��![]() �����
�����![]() ��
��
[��չ����]��5��������������ˮ������ʯ��ˮ�����������ɵĶ�����̼��ȫ���գ�����Dװ����װ���������ƹ����U�ιܣ�������װ�г���ʯ��ˮ��ϴ��ƿ�����������������ˮ������ʯ��ˮ�����������ɵĶ�����̼��ȫ���գ�CO2+Ca��OH��2=CaCO3��+H2O��
��6�������Բ��ó��������ⶨ��Ʒ�Ĵ��ȣ���ȡһ�������ռ���Ʒ��ȫ�ܽ���ˮ�У�Ȼ������Һ����μ����Ȼ�����Һ��ֱ�����ٲ�������Ϊֹ�����ˡ�ϴ�ӡ��������������������Ȼ���������ɵ�̼�ᱵ���������������û�ѧ����ʽNa2CO3+BaCl2=BaCO3��+2NaCl�����̼���Ƶ��������������ռ���Ʒ���������ɼ�����䴿�ȣ����ȡһ�������ռ���Ʒ��ȫ�ܽ���ˮ�У�Ȼ������Һ����μ����Ȼ�����Һ��ֱ�����ٲ�������Ϊֹ�����ˡ�ϴ�ӡ��������������������Ȼ���������ɵ�̼�ᱵ���������������û�ѧ����ʽNa2CO3+BaCl2=BaCO3��+2NaCl�����̼���Ƶ��������������ռ���Ʒ���������ɼ�����䴿��.