��Ŀ����
 ijѧϰС��ͬѧ����ͼ�Լ��еĹ���������Һ��ȡ������ȡ����Һ17g����������MnO2��ַ�Ӧ��������з��������㣺
ijѧϰС��ͬѧ����ͼ�Լ��еĹ���������Һ��ȡ������ȡ����Һ17g����������MnO2��ַ�Ӧ��������з��������㣺��1��С�����ݹ�����������Ԫ�ص������������������������������ʽΪ����������������=17g��5%��
| �������ԭ��������2 |
| �����������Է������� |
| 32 |
| 34 |
��ʦָ��С���ļ����Ǵ���ģ�����Ϊ�����ԭ����
A�������������Է���������������
B�����������е���Ԫ��û��ȫ��ת�Ƶ�������
��2��������ݻ�ѧ����ʽ������ȡ������������
���������ݹ���������Һ��ȡ�����ķ�Ӧ��ѧ����ʽ���з�������⣮
����⣺��1������������Һ��ȡ�����ķ�Ӧ��ѧ����ʽΪ��2H2O2
2H2O+O2�����ɴ˿�֪���������е���ԭ��û��ȫ��������������һ���ִ���ˮ�У����С�����ݹ�����������Ԫ�ص����������������������������Ǵ���ģ���ѡB��
��2������ȡ����������ΪX
2H2O2
2H2O+O2��
68 32
17g��5% X
=
X=0.4g
����ȡ����������Ϊ0.4g��
| ||
��2������ȡ����������ΪX
2H2O2
| ||
68 32
17g��5% X
| 68 |
| 17��5% |
| 32 |
| X |
X=0.4g
����ȡ����������Ϊ0.4g��
���������⿼��ѧ�����ݹ���������Һ��ȡ�����ķ�Ӧ��ѧ����ʽ���Ⲣ���м����������

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
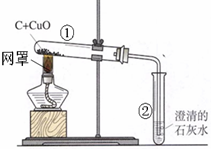 ijѧϰС��ͬѧ����ͼװ��̽��̼��ԭ����ͭ�Ĺ��̣�
ijѧϰС��ͬѧ����ͼװ��̽��̼��ԭ����ͭ�Ĺ��̣�
 ×100%=17g×5%×
×100%=17g×5%× ×100%��
×100%��