��Ŀ����
��ȥ��10�·����������ϳ������꣬���س��ֲ�ͬ�̶ȵĺ��飬���ϸ���������Ϊ���⺵��ʵʩ�˹����꣮
��1���˹������г��ù���ɱ������ǹ�̬�Ķ�����̼�����û�ѧ����ʽ��ʾ�������ʵ�����Ʒ�________���ճ���������ˮ�������ԣ���ԭ����________��
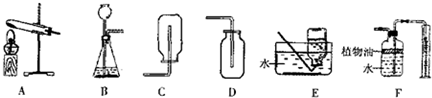
��2��ij��ѧ��ȤС��������ͼ��ʾװ������ȡ�����壮

��ʵ������ȡ������̼����ѡ�õķ���װ�ú��ռ�װ�÷ֱ�Ϊ________��________���ø÷���װ����ȡ����Ӧ�����������________��ѡ�������ռ�װ��Ҫ���ǵ�������________����д��һ�����ø���װ����ȡ��һ������Ļ�ѧ����ʽ________��
��Fװ�ÿ������������ɵ�CO2����������������ˮ���Ϸ�һ��ֲ���͵�Ŀ����________��ֲ�����Ϸ�ԭ�еĿ�����ʵ��Ľ��________����С���û�С�������Ӱ�죮
��3��С��ͬѧ������ijʯ��ʯ����ȡ������̼��Ϊ�ⶨ��ʯ��ʯ��̼��Ƶ��������������ʲ����ᷴӦ������6.0gʯ��ʯ��Ʒ���μ�ϡ���������ٲ������ݣ������ɶ�����̼����2.2g���Լ��㣺��ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ���٣�
�⣺��1��̼��ƺ����᳣���·�Ӧ�����Ȼ��ơ�������̼�����ˮ��CaCO3+2HCl=CaCl2+H2O+CO2����
��Ϊ������̼��ˮ��Ӧ����̼�ᣬ�����ԣ����ճ���������ˮ�������ԣ�
��2����ʵ������ȡ������̼����ѡ�õķ���װ�õ��ص����ڹ����Һ�峣�����Ʊ����壬���Է���װ��ѡ��B��
������̼������ܶȴ��ڿ��������Կ��������ſ������ռ���ѡ��װ��D����Ϊ������ˮ�����ܲ�����ˮ���ռ���
��ʵ��������ù����������������ڷ�Ӧ�У���������ֽ����ˮ����������һ��Ӧ�ķ���װ�õ��ص����ڹ����Һ�峣�����Ʊ����壻�������ܶȴ��ڿ��������Կ��������ſ������ռ���������ø���װ����ȡ�����Ļ�ѧ����ʽ�ɱ�ʾΪ��2H2O2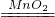 2H2O+O2����
2H2O+O2����
��ͼF�Dz�����ˮ���������̼�����������Ͳ����ˮ������Ӷ��������̼�������������̼������ˮ������Ҫ��ˮ���Ϸ���һ��ֲ���ͣ���ֹ������̼����ˮ����ˮ�������������ԭ���������ų�ˮ������ͽ���װ���е�����������ȵ�ԭ�����еģ�������û�ж�����̼����ʱ��ʢˮ�ļ���ƿ��ѹǿ��������ѹ����ȣ����ж�����̼��������ʱ������ƿ������ѹǿ����ˮ�ų����������Ϸ��Ŀ�����ʵ����û��Ӱ�죻
��3���⣺��μӷ�Ӧ��̼��Ƶ�����Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 2.2g
 =
=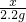 x=5g
x=5g
 ��100%=83.3%
��100%=83.3%
�𣺸�ʯ��ʯ��̼��Ƶ���������Ϊ83.3%��
�ʴ�Ϊ����1��CaCO3+2HCl=CaCl2+H2O+CO2����������̼��ˮ��Ӧ����̼�
��2����B��D�������Һ���ڳ����·�Ӧ��������ܶȺ��ܽ��ԣ�2H2O2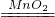 2H2O+O2��
2H2O+O2��
�ڷ�ֹ��CO2 ����ˮ������������������ȷ��û�У�
��3���⣺��μӷ�Ӧ��̼��Ƶ�����Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 2.2g
 =
=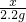 x=5g
x=5g
 ��100%=83.3%
��100%=83.3%
�𣺸�ʯ��ʯ��̼��Ƶ���������Ϊ83.3%��
��������1��ʵ������ȡ������̼��ʯ��ʯ��ϡ���ᣬ��̼��ƺ����᳣���·�Ӧ�����Ȼ��ơ�������̼�����ˮ�����ݶ�����̼��ˮ��Ӧ�IJ��������
��2���ٸ��ݷ�Ӧ���״̬�ͷ�Ӧ����ѡ����װ�ã�����������ܽ��Ժ��ܶ�ѡ���ռ�������
�ڸ���ʵ��Ŀ�ļ�������̼��ˮ���Խ��з���ֲ���͵����ã�������ˮ��������������ԭ���жϿ�����Ӱ�죻
��3�����ݻ�ѧ����ʽ���м��㣬���ݶ�����̼�����������̼��Ƶ����������������̼��Ƶ�����������
������������Ҫ����ѧ����ʵ�����Ʊ������Ͷ�����̼�ķ�Ӧԭ��������װ�õ�ѡ���ռ�װ�õ�ѡ���й���������ⶨ���������������
��Ϊ������̼��ˮ��Ӧ����̼�ᣬ�����ԣ����ճ���������ˮ�������ԣ�
��2����ʵ������ȡ������̼����ѡ�õķ���װ�õ��ص����ڹ����Һ�峣�����Ʊ����壬���Է���װ��ѡ��B��
������̼������ܶȴ��ڿ��������Կ��������ſ������ռ���ѡ��װ��D����Ϊ������ˮ�����ܲ�����ˮ���ռ���
��ʵ��������ù����������������ڷ�Ӧ�У���������ֽ����ˮ����������һ��Ӧ�ķ���װ�õ��ص����ڹ����Һ�峣�����Ʊ����壻�������ܶȴ��ڿ��������Կ��������ſ������ռ���������ø���װ����ȡ�����Ļ�ѧ����ʽ�ɱ�ʾΪ��2H2O2
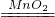 2H2O+O2����
2H2O+O2������ͼF�Dz�����ˮ���������̼�����������Ͳ����ˮ������Ӷ��������̼�������������̼������ˮ������Ҫ��ˮ���Ϸ���һ��ֲ���ͣ���ֹ������̼����ˮ����ˮ�������������ԭ���������ų�ˮ������ͽ���װ���е�����������ȵ�ԭ�����еģ�������û�ж�����̼����ʱ��ʢˮ�ļ���ƿ��ѹǿ��������ѹ����ȣ����ж�����̼��������ʱ������ƿ������ѹǿ����ˮ�ų����������Ϸ��Ŀ�����ʵ����û��Ӱ�죻
��3���⣺��μӷ�Ӧ��̼��Ƶ�����Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 2.2g
 =
=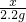 x=5g
x=5g ��100%=83.3%
��100%=83.3%�𣺸�ʯ��ʯ��̼��Ƶ���������Ϊ83.3%��
�ʴ�Ϊ����1��CaCO3+2HCl=CaCl2+H2O+CO2����������̼��ˮ��Ӧ����̼�
��2����B��D�������Һ���ڳ����·�Ӧ��������ܶȺ��ܽ��ԣ�2H2O2
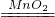 2H2O+O2��
2H2O+O2���ڷ�ֹ��CO2 ����ˮ������������������ȷ��û�У�
��3���⣺��μӷ�Ӧ��̼��Ƶ�����Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 2.2g
 =
=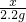 x=5g
x=5g ��100%=83.3%
��100%=83.3%�𣺸�ʯ��ʯ��̼��Ƶ���������Ϊ83.3%��
��������1��ʵ������ȡ������̼��ʯ��ʯ��ϡ���ᣬ��̼��ƺ����᳣���·�Ӧ�����Ȼ��ơ�������̼�����ˮ�����ݶ�����̼��ˮ��Ӧ�IJ��������
��2���ٸ��ݷ�Ӧ���״̬�ͷ�Ӧ����ѡ����װ�ã�����������ܽ��Ժ��ܶ�ѡ���ռ�������
�ڸ���ʵ��Ŀ�ļ�������̼��ˮ���Խ��з���ֲ���͵����ã�������ˮ��������������ԭ���жϿ�����Ӱ�죻
��3�����ݻ�ѧ����ʽ���м��㣬���ݶ�����̼�����������̼��Ƶ����������������̼��Ƶ�����������
������������Ҫ����ѧ����ʵ�����Ʊ������Ͷ�����̼�ķ�Ӧԭ��������װ�õ�ѡ���ռ�װ�õ�ѡ���й���������ⶨ���������������

��ϰ��ϵ�д�
�����Ŀ