��Ŀ����
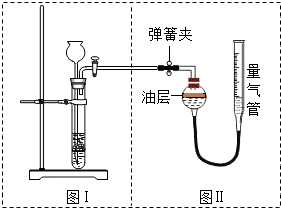 �ס�������ʵ��С��ֱ���С�Na2CO3��NaCl�������Na2CO3�����ⶨ����ʵ�飺
�ס�������ʵ��С��ֱ���С�Na2CO3��NaCl�������Na2CO3�����ⶨ����ʵ�飺
��1�����ͼ�������Եķ�����______��
��2������ķ����ǣ�����֪Ũ������μӵ�һ�������Ļ�����������������Һ��������ⶨ̼���Ƶĺ�������ʵ������г����ᡢ������ˮ���⣬�жϷ�Ӧ�Ƿ���ȫ�����õ����Լ���______��
��3������ķ����ǣ�����Ʒ��ϡ���ᷴӦ���ⶨ��Ӧ������CO2������������㲢�������Ʒ��Na2CO3������������ͼװ�òⶨ������CO2���������������ͼ��װ�����Ͳ��������______��ʹ��Ӧǰ���Ͳ��Ϸ�����ѹǿ��������ѹ��ͬ�IJ���������______��
��4����200mL�ܶ�Ϊ1.2g?mL-1��������Ϊ38%��Ũ���ᣬ����10%�ܶ�Ϊ1.02g?mL-1��ϡ���ᣬ��Ҫˮ______mL��
��5����ȡ��Na2CO3��NaCl�Ļ����6.2g������ijŨ�ȵ�ϡ����96gǡ�÷�Ӧ��ͼ���ռ���������̼1100mL����ʱ������̼���ܶ�Ϊ2g?L-1������ռ���������̼���ٿˣ��ڻ������̼���Ƶ������۷�Ӧ����Һ�����ʵ��������������۱���3λ��Ч���֣�
��6�����������һ����ס����������ͬ��ʵ�鷽�����ⶨ������е�Na2CO3��������ѡ�õ�ҩƷ��______��
�⣺��1������װ��һ�����Һ�壬����Һ�����м��������ԣ�
�ʴ�Ϊ��װ���м�ˮ�����ϵ��ɼУ���������ܣ�����Һ���
��2��̼���Ƶ���Һ�Լ��ԣ���ʹ��̪��죬������һָʾ�����жϷ�Ӧ�ķ��������
�ʴ�Ϊ��ָʾ�������̪�ȣ�
��3��Ϊ��֤�ռ��Ķ�����̼����ȷ����ֹ������̼����ˮ������Һ����ò��ܽ������̼���ͽ����ռ���
�ʴ�Ϊ����ֹ������̼����ˮ�����ɼУ��ƶ������ܣ�ʹ����Һ�洦��ͬһˮƽ�森
��4������ϡ��ǰ�����ʵ������������������ˮ�������x
 ��100%=10%
��100%=10%
���x=672ml
��5��������̼�������ǣ�0.11L��2g?L-1=2.2g����̼���������y�����ɵ��Ȼ���������z
Na2CO3+2HCl=2NaCl+H2O+CO2��
106 117 44
y z 2.2g
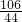 =
=
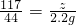
y=5.3g z=5.85g
ԭ��������Ȼ��Ƶ�����=6.2g-5.3g=0.9g
��Ӧ�����ʵ���������= ��100%=6.75%
��100%=6.75%
�ʴ�Ϊ��5.3g��5.85g��6.75%��
��6��̼����Ҳ��ͨ��ת���ɳ������Բ��������ݸ��ֽⷴӦ�ķ���������ɣ�
�ʴ�Ϊ��CaCl2��
��������1�������ܱ��ԽϺ�ʱ�����Һ�������
��2��̼������Һ�Լ��Կɽ�����̪�жϷ�Ӧ�Ľ��������
��3�����ݶ�����̼������ˮ���������ͽ������������Һ���ʱ�������ѹ��ȣ�
��4������ϡ��ǰ�����ʵ�������������
��5�����ö�����̼��������������̼�������������û�ѧ����ʽ����̼���Ƶ���������Ӧ�����Ȼ��Ƶ���ҺӦע���Ȼ��Ƴ����ɵ����ԭ������д��ڣ�
��6�����ݲ���ԭ����֪̼���ƿ���ͨ��ת���ɶ�����̼������ͬʱҲ��ͨ��ת����̼���εij������в�����
������������һ��ʵ���ۺ��⣬�漰��ʵ��Ļ����������ݻ�ѧ����ʽ�ļ��㣬��һ��ѵ��ѧ���������������ĺ��⣮
�ʴ�Ϊ��װ���м�ˮ�����ϵ��ɼУ���������ܣ�����Һ���
��2��̼���Ƶ���Һ�Լ��ԣ���ʹ��̪��죬������һָʾ�����жϷ�Ӧ�ķ��������
�ʴ�Ϊ��ָʾ�������̪�ȣ�
��3��Ϊ��֤�ռ��Ķ�����̼����ȷ����ֹ������̼����ˮ������Һ����ò��ܽ������̼���ͽ����ռ���
�ʴ�Ϊ����ֹ������̼����ˮ�����ɼУ��ƶ������ܣ�ʹ����Һ�洦��ͬһˮƽ�森
��4������ϡ��ǰ�����ʵ������������������ˮ�������x
 ��100%=10%
��100%=10%���x=672ml
��5��������̼�������ǣ�0.11L��2g?L-1=2.2g����̼���������y�����ɵ��Ȼ���������z
Na2CO3+2HCl=2NaCl+H2O+CO2��
106 117 44
y z 2.2g
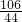 =
=
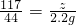
y=5.3g z=5.85g
ԭ��������Ȼ��Ƶ�����=6.2g-5.3g=0.9g
��Ӧ�����ʵ���������=
 ��100%=6.75%
��100%=6.75%�ʴ�Ϊ��5.3g��5.85g��6.75%��
��6��̼����Ҳ��ͨ��ת���ɳ������Բ��������ݸ��ֽⷴӦ�ķ���������ɣ�
�ʴ�Ϊ��CaCl2��
��������1�������ܱ��ԽϺ�ʱ�����Һ�������
��2��̼������Һ�Լ��Կɽ�����̪�жϷ�Ӧ�Ľ��������
��3�����ݶ�����̼������ˮ���������ͽ������������Һ���ʱ�������ѹ��ȣ�
��4������ϡ��ǰ�����ʵ�������������
��5�����ö�����̼��������������̼�������������û�ѧ����ʽ����̼���Ƶ���������Ӧ�����Ȼ��Ƶ���ҺӦע���Ȼ��Ƴ����ɵ����ԭ������д��ڣ�
��6�����ݲ���ԭ����֪̼���ƿ���ͨ��ת���ɶ�����̼������ͬʱҲ��ͨ��ת����̼���εij������в�����
������������һ��ʵ���ۺ��⣬�漰��ʵ��Ļ����������ݻ�ѧ����ʽ�ļ��㣬��һ��ѵ��ѧ���������������ĺ��⣮

��ϰ��ϵ�д�
 ��������ϵ�д�
��������ϵ�д�
�����Ŀ
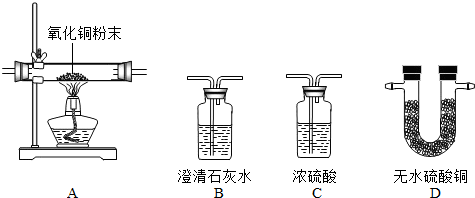
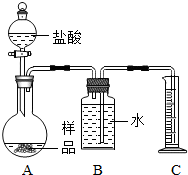 ��2013?�ٳ���ģ�⣩�ס�������ʵ��С��ֱ���С��ⶨNa2CO3��NaCl�������Na2CO3��������ʵ�飮
��2013?�ٳ���ģ�⣩�ס�������ʵ��С��ֱ���С��ⶨNa2CO3��NaCl�������Na2CO3��������ʵ�飮
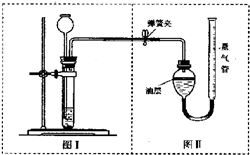 �ס�������ʵ��С��ֱ���С�Na2CO3��NaCl�������Na2CO3�����ⶨ����ʵ�飺
�ס�������ʵ��С��ֱ���С�Na2CO3��NaCl�������Na2CO3�����ⶨ����ʵ�飺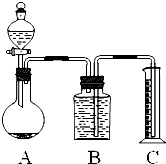 �ס�������ʵ��С��ֱ���С��ⶨNa2CO3��NaCl�������Na2CO3��������ʵ�飮
�ס�������ʵ��С��ֱ���С��ⶨNa2CO3��NaCl�������Na2CO3��������ʵ�飮