��Ŀ����
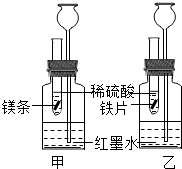 ��2012?������һģ��ij��ѧ�С��̽�������ķ�Ӧ���ɣ�
��2012?������һģ��ij��ѧ�С��̽�������ķ�Ӧ���ɣ������������͵�����������ϡ���ᣬ���з�Ӧ����ʽΪ
Mg+H2SO4=MgSO4+H2��
Mg+H2SO4=MgSO4+H2��
����1�����Ƚ�þ�����Ľ�����ԣ��۲쵽��������
þ��ϡ����������ݵ����ʱ�����ϡ����������ݵ����ʿ�
þ��ϡ����������ݵ����ʱ�����ϡ����������ݵ����ʿ�
����2�����Ƚ���ͬʱ����þ������ϡ���ᷴӦ�ų������Ķ��٣�����©���е�������
��װ�ó���©���е�Һ�����װ���еĸ�
��װ�ó���©���е�Һ�����װ���еĸ�
����3����ʵ�����õĽ��������������װ���в�����H2������
����
����
������ڡ�����С�ڡ����ڡ�����װ���в�����H2������[̽����]������ͭ�����������Ļ����Һ�м���һ������þ�ۣ���ַ�Ӧ����ˣ����˳��Ĺ����еμ�ϡ���ᣬû�����ݲ����������ж���ȷ����
ACD
ACD
��A���˳��Ĺ�����һ������ͭ
B���˳��Ĺ�����ܺ�������þ
C����Һ�п��ܺ�������ͭ
D����Һ��һ����������þ������������
������[̽��һ]
����þ�����ᷴӦԭ����д����ʽ��
��1������þ�����Ľ������ǿ���������۲쵽������
��2�����ݽ����Ļ�Լ��������ᷴӦ�������仯�������
��3�����ݻ�ѧ��Ӧǰ��Ԫ�ص������غ�������
[̽����]
�ɸ��ݽ����˳�����ʵĻ�ѧ���ʣ����ȴ��˳��Ĺ��岻�ܺ����ᷴӦ�����жϣ���δӽ����˳�����и�����Ϣ��һ������þ�ۡ������жϣ�
����þ�����ᷴӦԭ����д����ʽ��
��1������þ�����Ľ������ǿ���������۲쵽������
��2�����ݽ����Ļ�Լ��������ᷴӦ�������仯�������
��3�����ݻ�ѧ��Ӧǰ��Ԫ�ص������غ�������
[̽����]
�ɸ��ݽ����˳�����ʵĻ�ѧ���ʣ����ȴ��˳��Ĺ��岻�ܺ����ᷴӦ�����жϣ���δӽ����˳�����и�����Ϣ��һ������þ�ۡ������жϣ�
����⣺[̽��һ]
þ�����ᷴӦ��������þ��������
�ʴ�Ϊ��
Mg+H2SO4=MgSO4+H2����
��1��þ�Ļ�Դ���������ˣ�þ��ϡ����������ݵ����ʱ�����ϡ����������ݵ����ʿ죻
�ʴ�Ϊ��
þ��ϡ����������ݵ����ʱ�����ϡ����������ݵ����ʿ죻
��2���������Խǿ�����ᷴӦԽ���ң�þ�Ļ�Դ���������λʱ����þ������Ӧ��������࣬�������ᷴӦ���ȣ�þ���ᷴӦ�ų������࣬����©����Һ�������ĸߣ�
�ʴ�Ϊ��
��װ�ó���©���е�Һ�����װ���еĸߣ�
��3��þ���������ᷴӦ��������������=��������Ԫ�ص��������������֪�����õĽ�����������˵��ϡ�����е���������ȫ���μӷ�Ӧ�����ס���װ�������ӵ�ϡ�����ǵ������͵�����������������װ��������������ȣ�������������Ԫ�ص�������ȣ�����������������ȣ�
�ʴ�Ϊ�����ڣ�
[̽����]
A�����ݽ����˳���֪þ�������ã�����ͭ���ã����Լ���þ�ۣ�����þ�ۺ��Ȼ�ͭ��Һ��Ӧ���û�������ͭ�������˳��Ĺ�����һ������ͭ����A��ȷ��
B���������⣬���˳��Ĺ����еμ�ϡ���ᣬû�����ݲ�����˵��������һ����������þ����B����
C����Ϊ�������һ������þ�ۣ���B��֪��þ�۲��㣬��������ͭʣ�࣬��C��ȷ��
D�����ڣ�����þ�۲��뷴Ӧ��������Һ��һ��������þ��������������˵����������δ��Ӧ��������Һ��Ҳһ����������������D��ȷ��
��ѡACD��
þ�����ᷴӦ��������þ��������
�ʴ�Ϊ��
Mg+H2SO4=MgSO4+H2����
��1��þ�Ļ�Դ���������ˣ�þ��ϡ����������ݵ����ʱ�����ϡ����������ݵ����ʿ죻
�ʴ�Ϊ��
þ��ϡ����������ݵ����ʱ�����ϡ����������ݵ����ʿ죻
��2���������Խǿ�����ᷴӦԽ���ң�þ�Ļ�Դ���������λʱ����þ������Ӧ��������࣬�������ᷴӦ���ȣ�þ���ᷴӦ�ų������࣬����©����Һ�������ĸߣ�
�ʴ�Ϊ��
��װ�ó���©���е�Һ�����װ���еĸߣ�
��3��þ���������ᷴӦ��������������=��������Ԫ�ص��������������֪�����õĽ�����������˵��ϡ�����е���������ȫ���μӷ�Ӧ�����ס���װ�������ӵ�ϡ�����ǵ������͵�����������������װ��������������ȣ�������������Ԫ�ص�������ȣ�����������������ȣ�
�ʴ�Ϊ�����ڣ�
[̽����]
A�����ݽ����˳���֪þ�������ã�����ͭ���ã����Լ���þ�ۣ�����þ�ۺ��Ȼ�ͭ��Һ��Ӧ���û�������ͭ�������˳��Ĺ�����һ������ͭ����A��ȷ��
B���������⣬���˳��Ĺ����еμ�ϡ���ᣬû�����ݲ�����˵��������һ����������þ����B����
C����Ϊ�������һ������þ�ۣ���B��֪��þ�۲��㣬��������ͭʣ�࣬��C��ȷ��
D�����ڣ�����þ�۲��뷴Ӧ��������Һ��һ��������þ��������������˵����������δ��Ӧ��������Һ��Ҳһ����������������D��ȷ��
��ѡACD��
�����������ѶȽϴ���Ҫ�����˽����Ļ�ѧ���ʡ�ѧ���Խ����˳����������Լ��붨������һ�����������������ȣ��������������գ�������ѧ���������⡢��������������

��ϰ��ϵ�д�
�����Ŀ

