��Ŀ����
��10�֣���Ҫ��ȡһЩ������������������ʾ�����˶���ʵ�顣
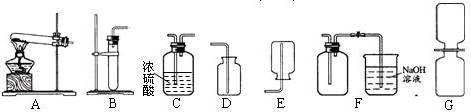
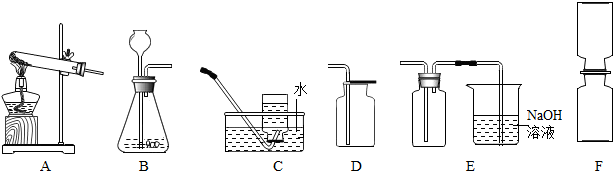
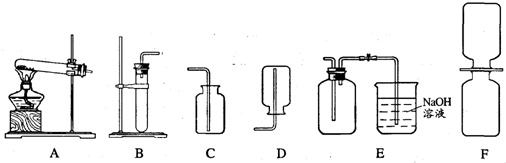
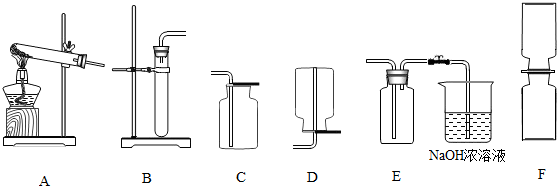
��֪���������������ͨ��ͭ��Ũ���ᷴӦ��Cu+4HN03(Ũ)��Cu(NO3)2+2N02��+2H2O�õ��� ����������һ�ֺ���ɫ�����壬�ܶȱȿ������ܺ�ˮ��Ӧ����HNO3��NO���壬NO��NO2���Ǵ�����Ⱦ�NO2�������Һ��Ӧ���ָ�������װ�ã�A��F����
(1)�ƶ��������ķ���װ�ÿ���ѡ ���ռ�����������װ�����ѡ ��
(2)д������������ˮ��Ӧ�Ļ�ѧ����ʽ ��
(3)Ҫ�õ�����Ķ��������ɽ���ͨ�� �������������ƣ�
(4)NOһ����O2�ͻ���NO2���ռ�NOӦ�� ����
(5)������ͼFװ����ʾ�����˶�ʱ��Ϊ��������ʾ��ʱ�䣬N02��÷��� ��ļ���ƿ�У���һƿ�ǿ�������ȥ��ƿ֮��IJ���Ƭ�����Կ���
��
(6)С��ͬѧ��װ��BC�����ȡ��һƿ��ɫ��ζ���������壬����ȡ�����������______��
������ķ�����֤����ȡ������____________________________________________��
(1)B E
(2)3NO2+H2O=2HNO3+NO ��2�֣�
(3)Ũ���� ��4����ˮ
��5���ϣ�����ɫ����Ʈ�£�����ƿ���ж������˺���ɫ���壬�����ƿ��������ɫ��ͬ��
��6��CO2 �� O2 ����ƿ�е������������ʯ��ˮ������ǡ���һ�������ǵ�ľ�����뼯��ƿ�ڣ�ľ����ȼ��
����

 ��У����ϵ�д�
��У����ϵ�д�