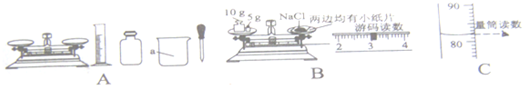
��Ŀ����
ijͬѧ����ȡ�ľ���������ͼ��ʾ���С�������������һ�����Ȼ�����Һ����ʵ�飺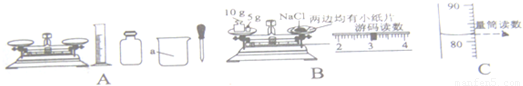
��1��ͼC�������������� ��
��2����ɴ�ʵ����ȷ�IJ���˳���ǣ� ������ţ������ܽ�ڰ����ƺõ���Һװ���Լ�ƿ�ۼ���ܳ���
��3����ʵ��Ҫ��ͼA�л�ȱ�ٵIJ��������� �������ƣ������� ��
��4��С溰�ͼB�IJ�����ȡ�Ȼ��ƣ�С����С溵IJ����д�������ΪС溲����еĴ����� ��
��5����˼������ȡ���Ȼ����к���ˮ�֣��������Ƶ���Һ�������������� ���ƫ����ƫС������Ӱ�족����
���𰸡���������1�����ݳ��������ش�
��2������������Һ�IJ���������
��3������������Һ��������;�Լ���Ҫ�����������ش�
��4��ʹ����ƽ�������ʵ�����Ӧ���������롯��
��5�������������������ļ��㷽������������������
����⣺��1��ͼC����������Ͳ��
��2��������Һ�IJ����ǣ����㡢�������ܽ⡢װƿ��
��3��������Һ��Ҫ�IJ��������У��ձ�����Ͳ����ͷ�ιܡ����������ձ����������������Ǽ��ٹ����ܽ⣻
��4��ʹ����ƽ�������ʵ�����Ӧ���������롯��ͼ��ͬѧ�IJ������÷ŷ��ˣ�
��5������ȡ���Ȼ����к���ˮ�֣���ʵ�ʳ������Ȼ��Ƶ�����С����Ҫ���Ȼ��Ƶ��������Ӷ�ʹ�����Ƶ���Һ��������������С��
�ʴ�Ϊ����1����Ͳ��
��2���ۢܢ٢ڣ�
��3�������������ٹ����ܽ⣻
��4�����뵹�û�����������̣��Ȼ��Ʒ������̣�
��5��ƫС��
������������Ҫ������������Һ��һ�㲽�裬������ʹ�ã�����������������Һ�������������仯���������֪ʶ����ϸ�������ɣ�
��2������������Һ�IJ���������
��3������������Һ��������;�Լ���Ҫ�����������ش�
��4��ʹ����ƽ�������ʵ�����Ӧ���������롯��
��5�������������������ļ��㷽������������������
����⣺��1��ͼC����������Ͳ��
��2��������Һ�IJ����ǣ����㡢�������ܽ⡢װƿ��
��3��������Һ��Ҫ�IJ��������У��ձ�����Ͳ����ͷ�ιܡ����������ձ����������������Ǽ��ٹ����ܽ⣻
��4��ʹ����ƽ�������ʵ�����Ӧ���������롯��ͼ��ͬѧ�IJ������÷ŷ��ˣ�
��5������ȡ���Ȼ����к���ˮ�֣���ʵ�ʳ������Ȼ��Ƶ�����С����Ҫ���Ȼ��Ƶ��������Ӷ�ʹ�����Ƶ���Һ��������������С��
�ʴ�Ϊ����1����Ͳ��
��2���ۢܢ٢ڣ�
��3�������������ٹ����ܽ⣻
��4�����뵹�û�����������̣��Ȼ��Ʒ������̣�
��5��ƫС��
������������Ҫ������������Һ��һ�㲽�裬������ʹ�ã�����������������Һ�������������仯���������֪ʶ����ϸ�������ɣ�

��ϰ��ϵ�д�
 �»����ܶ�Ա��ϵ�д�
�»����ܶ�Ա��ϵ�д� ����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
�����Ŀ