��Ŀ����
��2004?���ң���ͼΪij�Ӹ�ʳ�ΰ�װ�����ϵı�ǩ��С��ͨ�����з�������ʳ���еĸ�Ԫ�صĺ�������װ��ϡ�����С�ձ���������Ϊ200.00g������30.00g������Ʒ����ַ�Ӧ������ʣ�࣮��Ӧ���ձ�����Һ��������Ϊ229.67g������֪��CaCO3+2HNO3=Ca��NO3��2+H2O+CO2����
��1����Ӧ�����в���______g CO2
��2��30.00g��ʳ���к�̼��Ƶ������Ƕ��٣�______
��3���˼Ӹ�ʳ�θƺ����Ƿ���ϱ�ǩҪ��______��

���𰸡���������1�����������غ㶨�ɣ���Ӧǰ�����ʵ��������䣻���н��
��2�����ݻ�ѧ��Ӧ����ʽ���������ɶ�����̼���������м���̼��Ƶ�������
��3������30.00gʳ���к�̼��Ƶ������������Ԫ�ص�������������Ӹ�ʳ�θ�Ԫ�ص��������������ǩ�Ƚϣ����н��
����⣺��1�����������غ㶨�ɣ���Ӧ�����в���CO2������Ϊ��200.00g+30.00g-229.67g=0.33g
��2�����ʳ���к�̼��Ƶ�����Ϊx
CaCO3+2HNO3=Ca��NO3��2 +CO2��+H2O
100 44
x 0.33g
100��44=x��0.33g
x=0.75g
30.00gʳ���к�̼���0.75g��
��3���Ӹ�ʳ�θ�Ԫ�ص���������Ϊ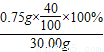 ×100%=1.0%��
×100%=1.0%��
���ݱ�ǩ�ƣ�1.3%��1.0%��0.3%
��� �˼Ӹ�ʳ�θƺ������ϱ�ǩҪ��
���������⿼��ѧ�����������غ㶨�ɣ���ѧ��Ӧ����ʽ�ļ��㣬���з��������������
��2�����ݻ�ѧ��Ӧ����ʽ���������ɶ�����̼���������м���̼��Ƶ�������
��3������30.00gʳ���к�̼��Ƶ������������Ԫ�ص�������������Ӹ�ʳ�θ�Ԫ�ص��������������ǩ�Ƚϣ����н��
����⣺��1�����������غ㶨�ɣ���Ӧ�����в���CO2������Ϊ��200.00g+30.00g-229.67g=0.33g
��2�����ʳ���к�̼��Ƶ�����Ϊx
CaCO3+2HNO3=Ca��NO3��2 +CO2��+H2O
100 44
x 0.33g
100��44=x��0.33g
x=0.75g
30.00gʳ���к�̼���0.75g��
��3���Ӹ�ʳ�θ�Ԫ�ص���������Ϊ
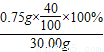 ×100%=1.0%��
×100%=1.0%�����ݱ�ǩ�ƣ�1.3%��1.0%��0.3%
��� �˼Ӹ�ʳ�θƺ������ϱ�ǩҪ��
���������⿼��ѧ�����������غ㶨�ɣ���ѧ��Ӧ����ʽ�ļ��㣬���з��������������

��ϰ��ϵ�д�
 Ʒѧ˫�ž�ϵ�д�
Ʒѧ˫�ž�ϵ�д� Сѧ��ĩ���100��ϵ�д�
Сѧ��ĩ���100��ϵ�д�
�����Ŀ
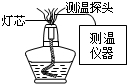 ��2004?���ң��ƾ��Ƶ�������ġ�����������������֣�Ϊ̽�������¶ȣ�������Ա������IJ���װ�ý���ʵ�飨��ͼ5����������£�̽ͷλ����ָ����̽ͷ���о�Ĵ�ֱ�߶ȣ���
��2004?���ң��ƾ��Ƶ�������ġ�����������������֣�Ϊ̽�������¶ȣ�������Ա������IJ���װ�ý���ʵ�飨��ͼ5����������£�̽ͷλ����ָ����̽ͷ���о�Ĵ�ֱ�߶ȣ���