��Ŀ����
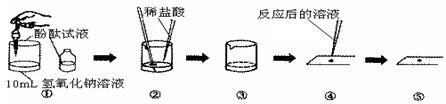
��ͼ����ͼ���кͷ�Ӧ��ʾ��ͼ
ʵ��������£�
����1�����ձ��м���10mL����������Һ���ٵ��˼��η�̪��Һ����ͼ�Т٣�
��1����ʱ�ձ�����Һ�� ɫ��
��2�������̪��Һ��Ŀ���� ��
����2�����õι���������ϡ���ᣬ�����Ͻ�����Һ������Һ��ɫǡ�ñ����ɫΪֹ����ͼ�Тڢۣ�
��3����һ����������������Һ��pH��α仯�� ��
��4���ò��������Ͻ�����Һ��Ŀ���� ��
��5�����ձ��У�������Ӧ�Ļ�ѧ����ʽ�� ��
����3��ȡ����������Ӧ�����ɫ��Һ���ڲ���Ƭ�ϣ��õ紵��ʹҺ��������
��6����������ڲ���Ƭ�ϵõ����Ǵ���������֤�����������Ȼ��ƶ������������ƣ�
�����ʵ�鲽������� ��
���𰸡���������̪��������Һ��죬����������������Ƿ����кͷ�Ӧ������м��ᣬ��Һ��PH�ɴ��С���ò���������ɼ�������кͣ������Ȼ��ƺ��������ƿ��÷�̪��Һ��
����⣺��1������Һʹ��̪��Һ��죬�ʴ�ʱ�ձ�����Һ�Ǻ�ɫ��
��2������к�ʱ�������̪��Һ��Ŀ������ʾ��Һ����ͼ��Ƿ�����Ӧ��
��3������м���ʱ����Һ�� PH�ɴ��С��
��4������к�ʱ���ò��������Ͻ�����Һ��Ŀ���� ʹ��ͼ��ַ�����ѧ��Ӧ��
��5���������ƺ����ᷴӦ�Ļ�ѧ����ʽ�� NaOH+HCl�TNaCl+H2O��
��6�������Ȼ��ƺ���������ʱ�ɸ�������Һ������Բ�ͬ�������ǣ�
ȡ����Ƭ�������������Թ��У���ˮ�ܽ⣬�����е�����ɫ��̪��Һ����Һ��Ϊ��ɫ��
�ʴ�Ϊ����1���죮
��2����ʾ��Һ����ͼ��Ƿ�����Ӧ��
��3��PH�ɴ��С��
��4��ʹ��ͼ��ַ�����ѧ��Ӧ��
��5��NaOH+HCl�TNaCl+H2O��
��6��ȡ����Ƭ�������������Թ��У���ˮ�ܽ⣬�����е�����ɫ��̪��Һ����Һ��Ϊ��ɫ��
���������⿼��������к͵IJ������̺�ԭ����Ҫע���̪��Һ�����ã�
����⣺��1������Һʹ��̪��Һ��죬�ʴ�ʱ�ձ�����Һ�Ǻ�ɫ��
��2������к�ʱ�������̪��Һ��Ŀ������ʾ��Һ����ͼ��Ƿ�����Ӧ��
��3������м���ʱ����Һ�� PH�ɴ��С��
��4������к�ʱ���ò��������Ͻ�����Һ��Ŀ���� ʹ��ͼ��ַ�����ѧ��Ӧ��
��5���������ƺ����ᷴӦ�Ļ�ѧ����ʽ�� NaOH+HCl�TNaCl+H2O��
��6�������Ȼ��ƺ���������ʱ�ɸ�������Һ������Բ�ͬ�������ǣ�
ȡ����Ƭ�������������Թ��У���ˮ�ܽ⣬�����е�����ɫ��̪��Һ����Һ��Ϊ��ɫ��
�ʴ�Ϊ����1���죮
��2����ʾ��Һ����ͼ��Ƿ�����Ӧ��
��3��PH�ɴ��С��
��4��ʹ��ͼ��ַ�����ѧ��Ӧ��
��5��NaOH+HCl�TNaCl+H2O��
��6��ȡ����Ƭ�������������Թ��У���ˮ�ܽ⣬�����е�����ɫ��̪��Һ����Һ��Ϊ��ɫ��
���������⿼��������к͵IJ������̺�ԭ����Ҫע���̪��Һ�����ã�

��ϰ��ϵ�д�
�����Ŀ