��Ŀ����
��ѧ�����ǵ�����ϢϢ��أ�
��1�����������������̼��С�մ��Ȼ��ơ�ˮ����������ѡ���ʵ������ʣ��û�ѧʽ��գ�������ܼ���________������������ͷʱʹ�÷��ͷ۵���Ҫ�ɷ���________���ܲ�������ЧӦ��������________��
��2������������Դ�������õ���Ҫ��չ����ѧ�����룬ˮ�ڴ������������·ֽ���Ƶ�������д���÷�Ӧ�Ļ�ѧ����ʽ��________��һ����̼�������������ͬ�Ļ�ѧ���ʣ���һ����̼ȴ��������Դ�ķ�չ���Ӱ�ȫ�ͻ����ĽǶ�����������Ҫԭ����________��
��3���״���CH3OH���ж���������ʹ�۾�ʧ�������������������о�֤���ð�����NH3���������м״��Ĺ�ҵ��ˮ����ת��Ϊ�������ʣ���ѧ����ʽΪ��5CH3OH+12O2+6NH3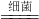 3X+5CO2+19H2O��NH3�е�Ԫ�صĻ��ϼ���________���״�����Ԫ�ص���������Ϊ________��X�Ļ�ѧʽ��________��
3X+5CO2+19H2O��NH3�е�Ԫ�صĻ��ϼ���________���״�����Ԫ�ص���������Ϊ________��X�Ļ�ѧʽ��________��
�⣺��1��ˮ��������ܼ���1��ˮ���Ӻ���2����ԭ�Ӻ�1����ԭ�ӣ���̼�����Ƴ�����ʳƷ���͵��У�1��̼�����Ʒ��Ӻ���1����ԭ�Ӻ�1��̼�������������̼���ܲ�������ЧӦ�����壬1��������̼���Ӻ���1��̼ԭ�Ӻ�2����ԭ�ӣ��ʴ�Ϊ��H2O��NaHCO3��CO2
��2��ˮ�ڴ������������·ֽ���Ƶ���������������ƽ���ɣ��Ӱ�ȫ�ͻ����ĽǶ�����������һ����̼����Ҫԭ���ǣ�һ����̼�ж���һ����̼��ȼ�ղ����Ƕ�����̼�����������ЧӦ���ʴ�Ϊ��2H2O 2H2��+O2���� һ����̼�ж���һ����̼��ȼ�ղ����Ƕ�����̼�����������ЧӦ��
2H2��+O2���� һ����̼�ж���һ����̼��ȼ�ղ����Ƕ�����̼�����������ЧӦ��
��3��NH3����Ԫ�صĻ��ϼ���+1�ۣ���Ԫ�صĻ��ϼ���-3�ۣ��״�����Ԫ�ص���������= =12.5%�����ݻ�ѧ��Ӧǰ��ԭ�ӵ��������Ŀ���䣬�ó�X�ǵ������ʴ�Ϊ��-3�ۣ�12.5%��N2
=12.5%�����ݻ�ѧ��Ӧǰ��ԭ�ӵ��������Ŀ���䣬�ó�X�ǵ������ʴ�Ϊ��-3�ۣ�12.5%��N2
������ˮ��������ܼ���̼�����Ƴ�����ʳƷ���͵��У�������̼���ܲ�������ЧӦ�����壮ˮ�ڴ������������·ֽ���Ƶ���������������ƽ���ɣ��Ӱ�ȫ�ͻ����ĽǶ�����������һ����̼����Ҫԭ���ǣ�һ����̼�ж���һ����̼��ȼ�ղ����Ƕ�����̼�����������ЧӦ��NH3����Ԫ�صĻ��ϼ���+1�ۣ���Ԫ�صĻ��ϼ���-3�ۣ��йصļ���Ҫȷ��
�����������㿼���˻�ѧʽ�ͻ�ѧ����ʽ����д��һ����̼�Ķ��Ժ������غ㶨�ɵȣ���Ϥ�������ʵ����ʣ��ܸ������ʵ������ƶ����ʵ���;��Ԫ�ط��š���ѧʽ����ѧ����ʽ�Ȼ�ѧ�������д���п�����Ҫ����֮һ��Ҫ��ǿ��ϰ������Ӧ�ã���������Ҫ������ѡ�����������У�
��2��ˮ�ڴ������������·ֽ���Ƶ���������������ƽ���ɣ��Ӱ�ȫ�ͻ����ĽǶ�����������һ����̼����Ҫԭ���ǣ�һ����̼�ж���һ����̼��ȼ�ղ����Ƕ�����̼�����������ЧӦ���ʴ�Ϊ��2H2O
 2H2��+O2���� һ����̼�ж���һ����̼��ȼ�ղ����Ƕ�����̼�����������ЧӦ��
2H2��+O2���� һ����̼�ж���һ����̼��ȼ�ղ����Ƕ�����̼�����������ЧӦ����3��NH3����Ԫ�صĻ��ϼ���+1�ۣ���Ԫ�صĻ��ϼ���-3�ۣ��״�����Ԫ�ص���������=
 =12.5%�����ݻ�ѧ��Ӧǰ��ԭ�ӵ��������Ŀ���䣬�ó�X�ǵ������ʴ�Ϊ��-3�ۣ�12.5%��N2
=12.5%�����ݻ�ѧ��Ӧǰ��ԭ�ӵ��������Ŀ���䣬�ó�X�ǵ������ʴ�Ϊ��-3�ۣ�12.5%��N2������ˮ��������ܼ���̼�����Ƴ�����ʳƷ���͵��У�������̼���ܲ�������ЧӦ�����壮ˮ�ڴ������������·ֽ���Ƶ���������������ƽ���ɣ��Ӱ�ȫ�ͻ����ĽǶ�����������һ����̼����Ҫԭ���ǣ�һ����̼�ж���һ����̼��ȼ�ղ����Ƕ�����̼�����������ЧӦ��NH3����Ԫ�صĻ��ϼ���+1�ۣ���Ԫ�صĻ��ϼ���-3�ۣ��йصļ���Ҫȷ��
�����������㿼���˻�ѧʽ�ͻ�ѧ����ʽ����д��һ����̼�Ķ��Ժ������غ㶨�ɵȣ���Ϥ�������ʵ����ʣ��ܸ������ʵ������ƶ����ʵ���;��Ԫ�ط��š���ѧʽ����ѧ����ʽ�Ȼ�ѧ�������д���п�����Ҫ����֮һ��Ҫ��ǿ��ϰ������Ӧ�ã���������Ҫ������ѡ�����������У�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ