��Ŀ����
27����A��ijУ��ѧ��ȤС���ͬѧѧϰ���ᡢ��ε�֪ʶ�Կα��С��ᡢ���֮�䲢���Ƕ��ܷ������ֽⷴӦ����仰��������Ȥ��չ����̽����ѧϰ��
��1����������⡿�������ֽⷴӦӦ�߱���Щ������
��2�����̽������С��ͬѧ������������ʵ�飬���־��ܷ�Ӧ����д���ܵĻ�ѧ����ʽ��
�����ᱵ��Һ��ϡ���� ��ϡ������̼�����Һ ��ϡ����������������Һ�����ᱵ��Һ��̼�����Һ��
��3�������۷�����������ӦΪʲô�ܹ�����������Ϊ����Щ��Ӧ�����Һ������������������ӣ�����������������˳����������ˮ���磺
������Ba 2++SO42-��BaSO4�� ������H ++CO32-��H2O+CO2��
������H ++OH-��H2O ������
��4�������۷�����������������С��ͬѧ�ó����½��ۣ����������Ƿ��г����������ˮ�����ж��ᡢ���֮���ܷ������ֽⷴӦ����Ҫ������
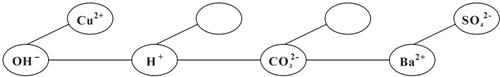
��5�����γ����硿����һ����˳������ijЩ���ӣ��Ϳ����γ�һ��֪ʶ���磮�������У��á�--�������ӵ����������Ӽ�������������ɳ����������ˮ��
����Ca 2+��HCO3-�������ӣ��뽫��������������ʵġ� ���ڣ�ʹ���γ�һ����Ϊ�����ĸ��ֽⷴӦ��֪ʶ���磮
���ڣ�ʹ���γ�һ����Ϊ�����ĸ��ֽⷴӦ��֪ʶ���磮
��B��̽����������

������̼�Ƿ��������ˮ������ֱ�ӹ۲쵽������ͨ��
��1����������⡿�������ֽⷴӦӦ�߱���Щ������
��2�����̽������С��ͬѧ������������ʵ�飬���־��ܷ�Ӧ����д���ܵĻ�ѧ����ʽ��
�����ᱵ��Һ��ϡ���� ��ϡ������̼�����Һ ��ϡ����������������Һ�����ᱵ��Һ��̼�����Һ��
Ba��NO3��2+K2CO3�TBaCO3��+2KNO3
����3�������۷�����������ӦΪʲô�ܹ�����������Ϊ����Щ��Ӧ�����Һ������������������ӣ�����������������˳����������ˮ���磺
������Ba 2++SO42-��BaSO4�� ������H ++CO32-��H2O+CO2��
������H ++OH-��H2O ������
Ba2+��CO32-
�������ӣ����Բŷ�����ѧ��Ӧ����4�������۷�����������������С��ͬѧ�ó����½��ۣ����������Ƿ��г����������ˮ�����ж��ᡢ���֮���ܷ������ֽⷴӦ����Ҫ������
��5�����γ����硿����һ����˳������ijЩ���ӣ��Ϳ����γ�һ��֪ʶ���磮�������У��á�--�������ӵ����������Ӽ�������������ɳ����������ˮ��
����Ca 2+��HCO3-�������ӣ��뽫��������������ʵġ�
 ���ڣ�ʹ���γ�һ����Ϊ�����ĸ��ֽⷴӦ��֪ʶ���磮
���ڣ�ʹ���γ�һ����Ϊ�����ĸ��ֽⷴӦ��֪ʶ���磮
��B��̽����������

������̼�Ƿ��������ˮ������ֱ�ӹ۲쵽������ͨ��
ѹǿ
�ı仯����Ӹ�֪��ͬ�����о����������ܽ���ˮʱ�����Ȼ��Ƿ��ȣ���������ѹǿ
�ı仯������U�ι���ˮλ�ı仯��ʹb
����a��b������ˮλ���ߵģ���������2�����̽���������ᱵ��Һ��̼�����Һ�����ݷ�Ӧ��������Ӧ������������д��ѧ����ʽ��
��3�������۷�����������ӦΪʲô�ܹ�����������Ϊ����Щ��Ӧ�����Һ������������������ӣ�����������������˳����������ˮ���磺������Ba 2++SO42-��BaSO4�� ������H ++CO32-��H2O+CO2�� ������H ++OH-��H2O ������ Ba2+��CO32-�������ӣ����Բŷ�����ѧ��Ӧ��
��5�����γ����硿����һ����˳������ijЩ���ӣ��Ϳ����γ�һ��֪ʶ���磮�������У��á�--�������ӵ����������Ӽ�������������ɳ����������ˮ�����ݷ�Ӧ�е����ӹ�ϵ������̽����֪ʶ�������绯���������֪ʶ����������ȱ���֣�
��B��̽����������
������̼�Ƿ��������ˮ������ֱ�ӹ۲쵽������ͨ�� ѹǿ�ı仯����Ӹ�֪��ͬ�����о����������ܽ���ˮʱ�����Ȼ��Ƿ��ȣ��������� ѹǿ�ı仯������U�ι���ˮλ�ı仯��ʹ b����ˮλ���ߵģ�
��3�������۷�����������ӦΪʲô�ܹ�����������Ϊ����Щ��Ӧ�����Һ������������������ӣ�����������������˳����������ˮ���磺������Ba 2++SO42-��BaSO4�� ������H ++CO32-��H2O+CO2�� ������H ++OH-��H2O ������ Ba2+��CO32-�������ӣ����Բŷ�����ѧ��Ӧ��
��5�����γ����硿����һ����˳������ijЩ���ӣ��Ϳ����γ�һ��֪ʶ���磮�������У��á�--�������ӵ����������Ӽ�������������ɳ����������ˮ�����ݷ�Ӧ�е����ӹ�ϵ������̽����֪ʶ�������绯���������֪ʶ����������ȱ���֣�
��B��̽����������
������̼�Ƿ��������ˮ������ֱ�ӹ۲쵽������ͨ�� ѹǿ�ı仯����Ӹ�֪��ͬ�����о����������ܽ���ˮʱ�����Ȼ��Ƿ��ȣ��������� ѹǿ�ı仯������U�ι���ˮλ�ı仯��ʹ b����ˮλ���ߵģ�
����⣺��2�����̽������С��ͬѧ������������ʵ�飬���־��ܷ�Ӧ����д���ܵĻ�ѧ����ʽ��
�����ᱵ��Һ��̼�����Һ��Ba��NO3��2+K2CO3�TBaCO3��+2KNO3��
��3�������۷�����������ӦΪʲô�ܹ�����������Ϊ����Щ��Ӧ�����Һ������������������ӣ�����������������˳����������ˮ���磺
������Ba 2++SO42-��BaSO4�� ������H ++CO32-��H2O+CO2��
������H ++OH-��H2O ������ Ba2+��CO32-�������ӣ����Բŷ�����ѧ��Ӧ��
��5�����γ����硿����һ����˳������ijЩ���ӣ��Ϳ����γ�һ��֪ʶ���磮�������У��á�--�������ӵ����������Ӽ�������������ɳ����������ˮ��
����Ca 2+��HCO3-�������ӣ�����������������ʵġ� ���ڣ�ʹ���γ�һ����Ϊ�����ĸ��ֽⷴӦ��֪ʶ���磮�ʣ�H+����HCO3-�γ����������̼��ˮ��CO32-����Ca2+�γ�̼��Ƴ�����
���ڣ�ʹ���γ�һ����Ϊ�����ĸ��ֽⷴӦ��֪ʶ���磮�ʣ�H+����HCO3-�γ����������̼��ˮ��CO32-����Ca2+�γ�̼��Ƴ�����
��B��̽����������

������̼�Ƿ��������ˮ������ֱ�ӹ۲쵽������ͨ�� ѹǿ�ı仯����Ӹ�֪��ͬ�����о����������ܽ���ˮʱ�����Ȼ��Ƿ��ȣ��������� ѹǿ�ı仯������U�ι���ˮλ�ı仯��ʹ b����ˮλ���ߵģ�
�ʴ�Ϊ����A����2��Ba��NO3��2+K2CO3�TBaCO3��+2KNO3
��3��Ba2+��CO32-����5��HCO3-��Ca2+
��B��ѹǿ������ƿ���һ���ɵ÷֣���ѹǿ��ƿ���������ͣ���һ���ɵ÷֣���b
�����ᱵ��Һ��̼�����Һ��Ba��NO3��2+K2CO3�TBaCO3��+2KNO3��
��3�������۷�����������ӦΪʲô�ܹ�����������Ϊ����Щ��Ӧ�����Һ������������������ӣ�����������������˳����������ˮ���磺
������Ba 2++SO42-��BaSO4�� ������H ++CO32-��H2O+CO2��
������H ++OH-��H2O ������ Ba2+��CO32-�������ӣ����Բŷ�����ѧ��Ӧ��
��5�����γ����硿����һ����˳������ijЩ���ӣ��Ϳ����γ�һ��֪ʶ���磮�������У��á�--�������ӵ����������Ӽ�������������ɳ����������ˮ��
����Ca 2+��HCO3-�������ӣ�����������������ʵġ�
 ���ڣ�ʹ���γ�һ����Ϊ�����ĸ��ֽⷴӦ��֪ʶ���磮�ʣ�H+����HCO3-�γ����������̼��ˮ��CO32-����Ca2+�γ�̼��Ƴ�����
���ڣ�ʹ���γ�һ����Ϊ�����ĸ��ֽⷴӦ��֪ʶ���磮�ʣ�H+����HCO3-�γ����������̼��ˮ��CO32-����Ca2+�γ�̼��Ƴ�������B��̽����������

������̼�Ƿ��������ˮ������ֱ�ӹ۲쵽������ͨ�� ѹǿ�ı仯����Ӹ�֪��ͬ�����о����������ܽ���ˮʱ�����Ȼ��Ƿ��ȣ��������� ѹǿ�ı仯������U�ι���ˮλ�ı仯��ʹ b����ˮλ���ߵģ�
�ʴ�Ϊ����A����2��Ba��NO3��2+K2CO3�TBaCO3��+2KNO3
��3��Ba2+��CO32-����5��HCO3-��Ca2+
��B��ѹǿ������ƿ���һ���ɵ÷֣���ѹǿ��ƿ���������ͣ���һ���ɵ÷֣���b
�������ᡢ�����Һ�䷢���ķ�Ӧ����ʵ������Һ�������γ��ѵ����������塢ˮ������Ĺ��̣�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ