��Ŀ����
�������м��������Ҵ��͵õ��Ҵ����ͣ�
��1���Ҵ���C2H5OH���ڿ����г��ȼ�յĻ�ѧ����ʽΪ
��2�������Ҵ����͵�����˵���в���ȷ����
A���Ҵ����ڿ���������Դ B���Ҵ����Ͳ�����������ȼ��
C��ʹ���Ҵ����Ϳɼ��ٴ�����Ⱦ D��ʹ���Ҵ����Ϳɽ�ʡʯ����Դ��
��1���Ҵ���C2H5OH���ڿ����г��ȼ�յĻ�ѧ����ʽΪ
C2H5OH+302
2C02+3H20
| ||
C2H5OH+302
2C02+3H20
��
| ||
��2�������Ҵ����͵�����˵���в���ȷ����
B
B
������ţ���A���Ҵ����ڿ���������Դ B���Ҵ����Ͳ�����������ȼ��
C��ʹ���Ҵ����Ϳɼ��ٴ�����Ⱦ D��ʹ���Ҵ����Ϳɽ�ʡʯ����Դ��
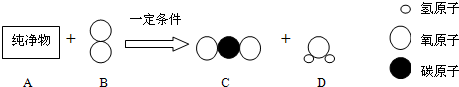
��������1���ɷ�Ӧ��������P��Ӧ�������������غ㶨�ɿ�����д��ѧ����ʽ��
��2���Ҵ����;����������м����Ҵ��Ƴɵģ����ڻ���
��2���Ҵ����;����������м����Ҵ��Ƴɵģ����ڻ���
����⣺��1���Ҵ����ȼ�������˶�����̼��ˮ����ѧ����ʽΪ��C2H5OH+302
2C02+3H20��
�ʴ�Ϊ����1��C2H5OH+302
2C02+3H20��
��2��A���Ҵ�����ͨ����ʳ�����Ƴɣ����ڿ�������Դ����A��ȷ��
B���Ҵ����Ϳ�����������ȼ�ϣ����ٷ������ŷţ���B����
C��ʹ���Ҵ������ܼ����к�������ŷţ���C��ȷ��
D���Ҵ�����ͨ����ʳ�����Ƴɣ�ʹ���Ҵ������ܽ�Լʯ����Դ����D��ȷ��
��ѡB��
| ||
�ʴ�Ϊ����1��C2H5OH+302
| ||
��2��A���Ҵ�����ͨ����ʳ�����Ƴɣ����ڿ�������Դ����A��ȷ��
B���Ҵ����Ϳ�����������ȼ�ϣ����ٷ������ŷţ���B����
C��ʹ���Ҵ������ܼ����к�������ŷţ���C��ȷ��
D���Ҵ�����ͨ����ʳ�����Ƴɣ�ʹ���Ҵ������ܽ�Լʯ����Դ����D��ȷ��
��ѡB��
���������⿼�����Ҵ������֪ʶ����д��ѧ����ʽʱҪ����ȷд����Ӧ���������Ļ�ѧʽ��Ȼ����ƽ��ע����Ӧ������

��ϰ��ϵ�д�
�����Ŀ