��Ŀ����
����Ŀ����ͥ��������һ����ѧС���磬�������Ų��ٻ�ѧ֪ʶ��
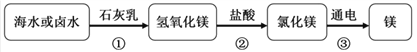
����Ȼ������Ҫ�ɷ��Ǽ��飨CH4������������________��ѡ�����л������������
����ȼ�յĻ�ѧ����ʽ��___________��0.2mol���������Լ��________��̼ԭ�ӡ�
������ʱ��ʳ�ν���ú�������ϣ������___ɫ��ÿ��ÿ��ʳ���������������6g���£�
��ij������ʳ��5.85g��������ʳ�ε����ʵ���Ϊ____mol��
��ͨ������ζ�ķ������������Ͼƺ�ʳ�ף��÷�����Ҫ�����˷��Ӿ���_____________�����ʡ�
��������װ��ˮ�����������ֱ��ˮ����ˮ���л���̿����____��ֱ��ˮΪ_____��ѡ��������������������
���𰸡� �л� CH4+2O2![]() CO2+2H2O 1.204��1023 �� 0.1 �����˶� ������ ���
CO2+2H2O 1.204��1023 �� 0.1 �����˶� ������ ���
��������������Ҫ���鳣���������;�����ʵļ���ľ̿�������ԣ�
��1�����飨CH4���к���̼Ԫ�أ��������л����������Ӧ�Ļ�ѧ����ʽ��֪��һ�������к���һ��̼ԭ������0.2mol���������Լ��1.204��1023��̼ԭ��
��2���Ƶ���ɫ��ӦΪ��ɫ��ʳ�ν���ú�������ϣ����Ի���ʻ�ɫ��1mol���Ȼ��Ƶ�����Ϊ58.5�ˣ���������ʳ��5.85g��������ʳ�ε����ʵ���Ϊ0.1mol��
��3���Ͼƺ�ʳ���лӷ��ԣ��Ƿ��Ӳ��ϵ��˶��Ľ����
��4������̿�������ԣ�����������������ֻ�ܳ��������ʡ�

����Ŀ���Ȼ���(NaCl) ������� (KNO3) �ڲ�ͬ�¶�ʱ���ܽ�ȼ����ܽ���������£�
�¶ȣ��棩 | 10 | 20 | 40 | 60 | 80 |
KNO3 | 20.9 | 31.6 | 63.9 | 110 | 169 |
NaCl | 35.8 | 36 | 36.6 | 37.3 | 38.4 |
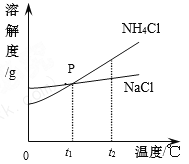
��ͼ���ܱ�ʾKNO3�ܽ�����ߵ���___����ס����ҡ�����
��������M������_______��
��Ҫ����100g 10%��KNO3��Һ����ҪKNO3����________g��
��t1oCʱ���������ļ��Ҷ������ʷֱ��ˮ�Ƴ���Ӧ�ı�����Һ���õ���Һ�����ϴ����_______����ס����ҡ�����
��20��ʱ���������ֱ�ʢ����ͬ����������غ��Ȼ��Ƶ��ձ��У�������100gˮ������ܽ�ָ���20�棬������ͼ��ʾ��
�����й�˵���У���ȷ����________��������ĸ��ţ�
A���ձ������ܽ����KNO3���ձ������ܽ����NaCl
B���ձ�������Һһ���Dz�������Һ
C�������¶Ȼ������ܼ����п��ܽ��ձ����й���ȫ���ܽ�
D�������ձ����е���Һ��Ϊ��������Һ����Һ��������������һ����С
����Ŀ����5g����M�������ᾧˮ���Σ����ֱ�Ͷ�뵽ʢ��10gˮ���ձ��У�����ܽ���й���δ�ܽ⡣����������±���ʾ��
�ձ���� | I | II | III |
δ�ܽ����������g�� | 2.8 | 1 | 0.4 |

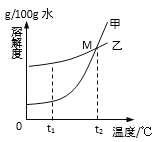
��20��ʱ��M��Һ��____��ѡ����͡��������͡�����Һ��
�������±�20��ʱ���ʵ��ܽ�ȿ����ж����ܽ��ԡ�M����_______�����ʡ�
�ܽ�ȣ�g/100gˮ�� | <0.01 | 0.01-1 | 1-10 | >10 |
�ܽ��� | ���� | �� | ���� | ���� |
���й��ձ�II���ձ�III�е�M��Һ��˵����ȷ����______��
A��30��ʱ��M���ܽ�Ƚϴ�
B���ձ�III����Һ���������ʽ϶�
C��M��Һ�����������������
D���ֱ�����20������Һ��������������M
��40��ʱ���ձ�III���ټ���35gˮ��M��Һ��������������Ϊ______��
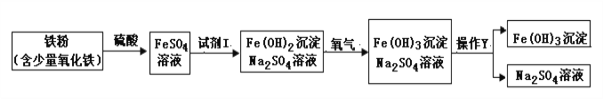
����Ŀ��ij������Ҫ�ɷ���NaCl�����ܻ���Na2CO3��Na2SO4��Ϊ�����������ʵijɷ֣�����������ʵ�飺
ȡ������Ʒ����ˮ�ܽ� | ����ϡ���� | ���������Ȼ��� | |
ʵ����� |
|
|
|
ʵ������ | ȫ���ܽ� | �����ݲ��� |
����ʵ����̣�Ԥ������ʵ�������Ҷ�Na2SO4�жϾ���ȷ����
A. �а�ɫ�������ɣ�һ����Na2SO4
B. ��û�г��������а�ɫ�������ɣ����ܺ���Na2SO4
C. �ް�ɫ�������ɣ�һ��û��Na2SO4
D. �������ް�ɫ�������ɣ���������Na2SO4