��Ŀ����
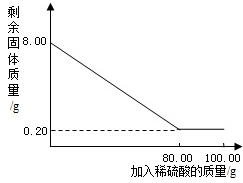 ��ͨ��״���£�������һ����ɫ����ζ�����壬������ˮ���ܶȱȿ���С��ʵ���ҳ���п����ϡ���ᷴӦ��ȡ������Zn+H2SO4�TZnSO4+H2����Ϊ�˵õ�0.20s������ijͬѧ������Ϊ8.00g�������ʵ�п����100.00g������ϡ���ᷴӦ����ͬѧ��õ��й�ʵ����������ͼ������ϵ�����ٶ����ʲ�����ˮ��Ҳ���μӷ�Ӧ����
��ͨ��״���£�������һ����ɫ����ζ�����壬������ˮ���ܶȱȿ���С��ʵ���ҳ���п����ϡ���ᷴӦ��ȡ������Zn+H2SO4�TZnSO4+H2����Ϊ�˵õ�0.20s������ijͬѧ������Ϊ8.00g�������ʵ�п����100.00g������ϡ���ᷴӦ����ͬѧ��õ��й�ʵ����������ͼ������ϵ�����ٶ����ʲ�����ˮ��Ҳ���μӷ�Ӧ������ش��������⣺
��1����ͬѧ������
��2��ʵ�ʲ��������������Ƕ��٣�����ȷ��0.01g��
��������1���������������ʽ���ռ�����ķ������ǣ�
��2����ͼʾ��֪����ȫ��Ӧ��ʣ�����ʵ�����Ϊ0.20g���ݴ˼����п��������Ȼ������п��ϡ���ᷴӦ�Ļ�ѧ����ʽ�Ͳ��뷴Ӧ��п���������Ϳɼ����ʵ�ʲ���������������
��2����ͼʾ��֪����ȫ��Ӧ��ʣ�����ʵ�����Ϊ0.20g���ݴ˼����п��������Ȼ������п��ϡ���ᷴӦ�Ļ�ѧ����ʽ�Ͳ��뷴Ӧ��п���������Ϳɼ����ʵ�ʲ���������������
����⣺��1���������֪������������ˮ���ʿ�����ˮ���ռ��������ܶȱȿ���С���ʿ��������ſ������ռ����ʴ�Ϊ����ˮ���������ſ�����
��2����ʵ����������������Ϊx��
�������֪��п�������ʵ�����Ϊ0.20g����ôп������Ϊ8.00g-0.20g�T7.80g��
Zn+H2SO4=ZnSO4+H2��
65 2
7.8g x
��
=
��
��֮�ã�x=0.24g��
��ʵ�ʲ���������������0.24g��
��2����ʵ����������������Ϊx��
�������֪��п�������ʵ�����Ϊ0.20g����ôп������Ϊ8.00g-0.20g�T7.80g��
Zn+H2SO4=ZnSO4+H2��
65 2
7.8g x
��
| 65 |
| 2 |
| 7.8g |
| x |
��֮�ã�x=0.24g��
��ʵ�ʲ���������������0.24g��
������������Ҫ����ѧ�����û�ѧ���̽��м����������ѧ��Ҫ��ȷʶͼ�ͷ���������ϵ����ͼ���ҳ���Ч���ݽ��м��㣬��Ҫ���������ݸ���˼ά��

��ϰ��ϵ�д�
�����Ŀ