��Ŀ����
��2003?�γǣ�ijͬѧΪ�˲ⶨ��������̼��Ƶ�������������ȡ5.55 g����ĺ������ĩ�������Ĵθ��¼��ȣ����ʲ��μӷ�Ӧ������ȴ������ʣ�������ظ���������¼������| �������� | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| ʣ�����������g�� | 4.25 | 3.95 | 3.35 | 3.35 |
��1����ȫ��Ӧ�����ɶ�����̼______g��
��2��������̼��Ƶ�����������
��3����ͬѧΪ�������о���Ҫ����ȡһ�������ĺ��������þ�ۻ�ϣ��ڿ����м�������Ӧ��ȫ�����ʲ��μӷ�Ӧ������÷�Ӧǰ���������䣬����ۺ�þ�۵������ȣ�
���𰸡���������1����ͼ����֪�������κ͵��Ĵ�ʣ������������ȣ�˵���������η�Ӧ������ȫ��Ӧ���������ɵ������ݳ������Ը��������غ㶨�ɡ��μӷ�Ӧǰ�����ʵ������ܺ͵��ڷ�Ӧ�����ɸ����ʵ������ܺ͡���֪����ȫ��Ӧ�����ɶ�����̼������=��Ӧǰ�������ĩ������-ʣ������������
��2������̼��Ƹ��¼��ȵĻ�ѧ����ʽ���ó�������֮��������ȣ��г�����ʽ������������뷴Ӧ��CaCO3��������Ȼ���������������ʽ���㼴�ɣ�
��3������CaCO3��Mg�ڿ����м��ȵĻ�ѧ����ʽ����֪������֪��CaCO3��Ӧ�ų���CO2����������Mg��Ӧ���յ�O2����������˸��ݷ���ʽ�ó���ʾCaCO3��Mg�������Ĵ���ʽ���ٽ��бȽϼ��ɣ�
����⣺��1����ȫ��Ӧ�����ɶ�����̼������Ϊ��5.55g-3.35g=2.2g��
�ʴ�Ϊ��2.2��
��2������뷴Ӧ��CaCO3������Ϊx��
CaCO3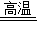 CaO+CO2��
CaO+CO2��
100 44
x 2.2g
��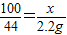
��֮�ã�x=5g��
��������̼��Ƶ���������Ϊ��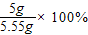 ��90.1%��
��90.1%��
�𣺺�������̼��Ƶ���������Ϊ90.1%��
��3��CaCO3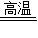 CaO+CO2����2Mg+O2=2MgO��
CaO+CO2����2Mg+O2=2MgO��
�۲췽��ʽ�ɵã���Ӧ�ų���CO2���������ڷ�Ӧ���յ�O2��������
����O2������=CO2������=X��
��ԭ��CaCO3������=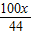 =
=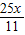 ��Mg������=
��Mg������=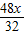 =1.5X��
=1.5X��
����Ϊ������CaCO390.1%�����Ժ��������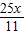 ÷90.1%��2.5X��
÷90.1%��2.5X��
���Ժ��������þ�۵�������Ϊ2.5x��1.5x=5��3��
�𣺺�����ۺ�þ�۵�������Ϊ5��3��
������������Ҫ����ѧ�����û�ѧ����ʽ�������غ㶨�ɽ��м����������
��2������̼��Ƹ��¼��ȵĻ�ѧ����ʽ���ó�������֮��������ȣ��г�����ʽ������������뷴Ӧ��CaCO3��������Ȼ���������������ʽ���㼴�ɣ�
��3������CaCO3��Mg�ڿ����м��ȵĻ�ѧ����ʽ����֪������֪��CaCO3��Ӧ�ų���CO2����������Mg��Ӧ���յ�O2����������˸��ݷ���ʽ�ó���ʾCaCO3��Mg�������Ĵ���ʽ���ٽ��бȽϼ��ɣ�
����⣺��1����ȫ��Ӧ�����ɶ�����̼������Ϊ��5.55g-3.35g=2.2g��
�ʴ�Ϊ��2.2��
��2������뷴Ӧ��CaCO3������Ϊx��
CaCO3
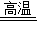 CaO+CO2��
CaO+CO2��100 44
x 2.2g
��
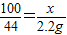
��֮�ã�x=5g��
��������̼��Ƶ���������Ϊ��
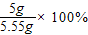 ��90.1%��
��90.1%���𣺺�������̼��Ƶ���������Ϊ90.1%��
��3��CaCO3
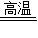 CaO+CO2����2Mg+O2=2MgO��
CaO+CO2����2Mg+O2=2MgO���۲췽��ʽ�ɵã���Ӧ�ų���CO2���������ڷ�Ӧ���յ�O2��������
����O2������=CO2������=X��
��ԭ��CaCO3������=
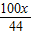 =
=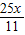 ��Mg������=
��Mg������=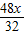 =1.5X��
=1.5X������Ϊ������CaCO390.1%�����Ժ��������
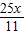 ÷90.1%��2.5X��
÷90.1%��2.5X�����Ժ��������þ�۵�������Ϊ2.5x��1.5x=5��3��
�𣺺�����ۺ�þ�۵�������Ϊ5��3��
������������Ҫ����ѧ�����û�ѧ����ʽ�������غ㶨�ɽ��м����������

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ