��Ŀ����
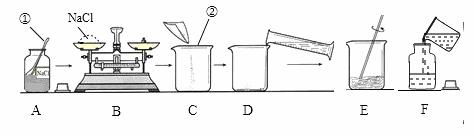
������50 g������������Ϊ15% ��NaCl ��Һ����ͼ��ijͬѧ������Һ�IJ������̡�
��Һ����ͼ��ijͬѧ������Һ�IJ������̡�
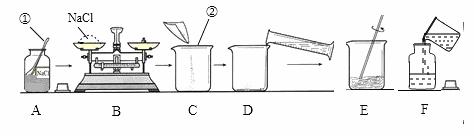
��1��д��������������ƣ���______________����_____________��
��2��B������Ӧ����NaCl��������________g��������ʱ�����Ȼ��Ʒŵ������̣�1 g���������룩����������û������ʵ�����Ƶ�NaCl��Һ����������������___________��
��3��������ȡˮ�����Ϊ__________��ˮ���ܶ�Ϊ1 g/cm3������Ͳ�Ĺ��ӦΪ___________���10 mL����50 mL������
��4��D����ʱ������ˮ���������ܵ���������������______���ƫ�ߡ���ƫ�͡�����Ӱ�족����ͬ����F��������Һ���䣬���ܵ���������������__________��
��1��ҩ�� �ձ� ��2��7.5 13.3% ��3�� 42.5 mL 50 mL
��4��ƫ�� ��Ӱ�� ��������1������ͼʾ��֪������ҩ�ף������ձ�����2������50 g 15% ���Ȼ�����Һ�������Ȼ��Ƶ�����Ϊ50 g��15%=7.5 g������ʱ�����Ȼ��Ʒŵ������̣��������Ȼ��Ƶ�ʵ������Ϊ7 g-0.5 g=6.5 g��ʵ�����Ƶ�NaCl��Һ����������������6.5 g/49 g  ��100%��13.3%����3������ˮ������Ϊ50 g-7.5 g=42.5 g�������Ϊ42.5 mL����Ͳ�Ĺ��ӦΪ50 mL����4�����ձ��е���ˮʱ������ˮ���������ܼ����٣���Һ��Ũ������õ���Һת��ƿ��ʱ�����������������������䡣
��100%��13.3%����3������ˮ������Ϊ50 g-7.5 g=42.5 g�������Ϊ42.5 mL����Ͳ�Ĺ��ӦΪ50 mL����4�����ձ��е���ˮʱ������ˮ���������ܼ����٣���Һ��Ũ������õ���Һת��ƿ��ʱ�����������������������䡣

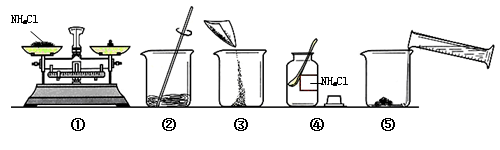
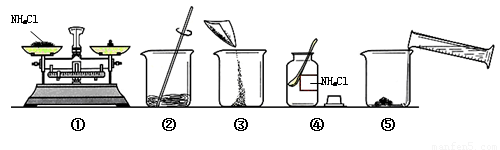
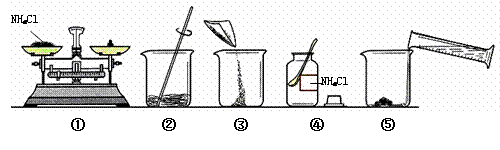
 ��Һ����ͼ��ijͬѧ������Һ�IJ������̡�
��Һ����ͼ��ijͬѧ������Һ�IJ������̡�