��Ŀ����
����NaNO3��AgNO3��HCl��Na2CO3��NaCl��Ca(NO3)2������Һ����Ca(NO3)2��Һƿ�����б�ǩ��Ϊȷ������������Һ����ʲô��������������ΪA��B��C��D��E��������ͽ���ʵ�飬����ʵ������(���±���ʾ)�ش�
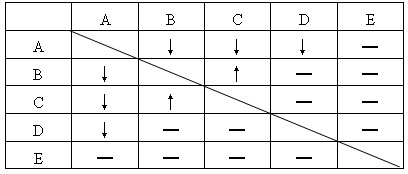
(1)����ʵ��������ȷ����A��D��E�Ļ�ѧʽ�����±���Ӧ�Ŀո��С�
A_________��D_________��E__________��
(2)Ϊ��ȷ��B��C���ʸ���ʲô����Ҫ��������ʵ�顣��ջش��й����⣺
�ٴ���ȷ�����Լ���ѡ��____________��Ϊ�����Լ����
����δȷ����B��C��Һ�зֱ�������ѡ�õļ����Լ����а�ɫ�������ɵ�ԭ��Һ��ѧʽ��__________��Ӧ�����ԭ��Һ��ѧʽ��__________��
��A+D��Ӧ�Ļ�ѧ����ʽ___________________��
A_________��D_________��E__________��
(2)Ϊ��ȷ��B��C���ʸ���ʲô����Ҫ��������ʵ�顣��ջش��й����⣺
�ٴ���ȷ�����Լ���ѡ��____________��Ϊ�����Լ����
����δȷ����B��C��Һ�зֱ�������ѡ�õļ����Լ����а�ɫ�������ɵ�ԭ��Һ��ѧʽ��__________��Ӧ�����ԭ��Һ��ѧʽ��__________��
��A+D��Ӧ�Ļ�ѧ����ʽ___________________��
��1��A��AgNO3��D��NaCl��E��NaNO3
��2����Ca(NO3)2
��Na2CO3��HCl
��AgNO3+NaCl==AgCl��+NaNO3
��2����Ca(NO3)2
��Na2CO3��HCl
��AgNO3+NaCl==AgCl��+NaNO3

��ϰ��ϵ�д�
 ��Ӣ���㿨ϵ�д�
��Ӣ���㿨ϵ�д� Ӧ����㲦ϵ�д�
Ӧ����㲦ϵ�д�
�����Ŀ
����NaNO3��AgNO3��HCl��Na2CO3��NaCl��Ca(NO3)2������Һ����Ca(NO3)2��Һƿ�����б�ǩ��Ϊȷ������������Һ����ʲô��������������ΪA��B��C��D��E��������ͽ���ʵ�飬����ʵ������(���±���ʾ)�ش�
| A | B | C | D | E | |
| A | �� | �� | �� | �� | |
| B | �� | �� | �� | �� | |
| C | �� | �� | �� | �� | |
| D | �� | �� | �� | �� | |
| E | �� | �� | �� | �� |
(1)����ʵ��������ȷ����A��D��E�Ļ�ѧʽ�����±���Ӧ�Ŀո��С�
A D E
(2)Ϊ��ȷ��B��C���ʸ���ʲô����Ҫ��������ʵ�顣��ջش��й����⣺
�ٴ���ȷ�����Լ���ѡ��____________��Ϊ�����Լ����
����δȷ����B��C��Һ�зֱ�������ѡ�õļ����Լ����а�ɫ�������ɵ�ԭ��Һ��ѧʽ��__________��Ӧ�����ԭ��Һ��ѧʽ��__________��
��A+D��Ӧ�Ļ�ѧ����ʽ ��