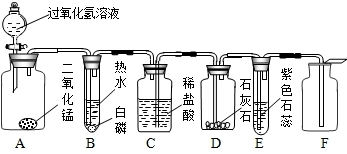
��Ŀ����
ijͬѧģ����������ѧ���ġ�������·�������������������ȡ��������֤�����ʵ�顣��Һ©��������A�г��ִ������ݣ�B�а���ȼ�գ�C��Һ���½����뿴ͼ�ش����⣺

A B C D E F
(1) A�з�Ӧ�Ļ�ѧ����ʽΪ�� ��
��2��B�а����ܹ�ȼ�յ�ԭ���� ��
(3) D�е�ʵ�������� ��
��Ӧ�Ļ�ѧ����ʽΪ ��
(4) E�е�ʵ�������� ��
��Ӧ�Ļ�ѧ����ʽΪ ��
(5) ��Fװ���ռ������������ ��
��6�����ô���ʯ��ϡ���ᷴӦ��ȡ������̼���壬��Ӧ�����Ķ�����̼���������������ϡ�����������ϵ��ͼ��ʾ������ʾ������ʯ�е����ʲ�������Ӧ��������ܽ���Բ��ƣ���ϡ���������ʵ����������Ƕ��٣�

��1��2H2O2 MnO2 2H2O + O2��
��2���������Ӵ��� �¶ȴﵽ�Ż�㡣
�¶ȴﵽ�Ż�㡣
��3��C��Һ�����D�У������ݲ����� CaCO3 + 2HCl���� CaCl2 + H2O + CO2��
��4�����ܿ�������ð������Һ����ɫ���ɫ�� CO2 + H 2O���� H2CO3
2O���� H2CO3
��5��������ܶȱȿ����ܶȴ�
��6��10%