��Ŀ����
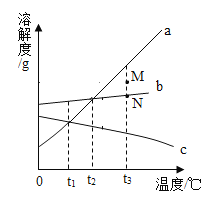
��������������ɱ�����ʵ��������һƿ���������������Ƶ�����������Ʒ,ij��ѧ��ȤС���ͬѧΪ�ⶨ����Ʒ��������������������,����������ʵ��:�ֱ�ȡ120 g��Ʒ��ƽ���ֳ�6�ݼ����ձ���,�ֱ����80gˮ�ܽ�,�ټ���̼������Һ��ַ�Ӧ,�Է�Ӧ��Ļ���������ͼ��ʾ�IJ���:
�������ʵ���������±���ʾ,�Լ���:(�������ԭ������Ϊ137)
ʵ������ | ��һ�� | �ڶ��� | ������ | ������ | ������ | ������ |
����̼������Һ������/g | 25 | 50 | 75 | 100 | 125 | 150 |
��ƽʾ��/g | 3.94 | 7.88 | 11.82 | 15.76 | m | 19.70 |
��1��m��ֵΪ______________��
��2����Ʒ��������������������____________��
��3����ǡ�÷�Ӧ��Һ�����Ƴ�������������Ϊ10%������������Һ�Ա�ʵ����ʹ��,��Ҫ�ٳ�ȡ____________���������ƹ���?
ij�������÷������Ʊ�K2SO4�Ĺ���������ͼ��ʾ,�������̻ش�������⡣
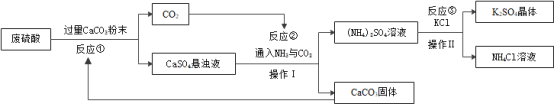
��1����Ӧ��֮ǰ��CaCO3�гɷ�ĩ��Ŀ����______________����Ӧ�ٵĻ�ѧ����ʽΪ___________��
��2��ʵ���Ҳ���IʱҪ�õ�һ�ֲ���������___________��
��3����Ӧ�ڵĻ�ѧ����ʽΪ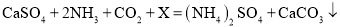 ,��X�Ļ�ѧʽΪ________________��
,��X�Ļ�ѧʽΪ________________��
��4����Ӧ����������ʵ��ܽ�����±���ʾ:
���� | (NH4)2SO4 | KCl | K2SO4 | NH4Cl |
�ܽ��/g(20�� C) | 75.4 | 34.2 | 11.1 | 37.2 |
��Ӧ���ڳ������ܷ�����ԭ����___________��
��5�����������п�ѭ��ʹ�õ�������_____________(�ѧʽ)��
ʵ�鷽����ȷ���ֱ�Ӿ�����ʵ���ܷ�ɹ�������ʵ�鷽���ܴﵽʵ��Ŀ�ĵ���
ѡ�� | ʵ��Ŀ�� | ʵ�鷽�� |
A | ������غͶ���������ȡ������ʣ�����л��ն������� | �ܽ⡢����,��ϴ�ӡ����� |
B | ����ľ̿��ĩ������ͭ��ĩ | �۲�������ɫ |
C | ����̼������Һ���Ƿ����Ȼ��� | �ӹ�����ϡ�����,�ٵμ���������Һ |
D | ��ȥ����������Һ�е������������� | ����������̼������Һ,���� |
A.A B.B C.C D.D



 B.��ʯ
B.��ʯ C.��
C.�� D.��Ȫˮ
D.��Ȫˮ