��Ŀ����
Ϊ������Դ����״��������ҳ����ʮ����滮��̨��
��1��ҳ��������Ȼ������Ҫ�ɷݶ��Ǽ��飬�����ڿ�������ȫȼ�յĻ�ѧ����ʽΪ��________����Ȼ����ú��________�ϳ�Ϊ����ʯȼ�ϣ�
��2��ҳ�����������ı��ҹ����ڵ���Դ��֣�Ϊ����������������Ӱ�죮���л���������ȼúû��ֱ�ӹ�ϵ����________������ĸ����
A�����ꡡ��B������ЧӦ������C���������ƻ�������D�����������������
��3������β���Ǵ�����Ⱦ��Ҫ��Դ֮һ������Ч��ת�������ɽ�����β�����ж����崦��Ϊ�����壬��ͼΪ�÷�Ӧ����ʾ��ͼ�� �ش��������⣮
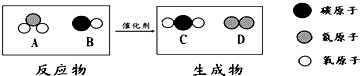
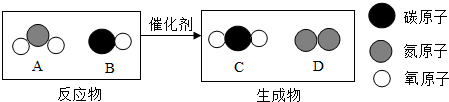
��A������Ԫ��������Ϊ________��
��4�������У����ڻ��������________����ͼ����ĸ����
���ڸ÷�Ӧ�У�����C��D��������Ϊ________������������������ȱ�ʾ����
�⣺��1�������ڿ�����ȼ�յ����ɶ�����̼��ˮ����Ӧ�Ļ�ѧ����ʽΪ��CH4+2O2  CO2+2H2O��
CO2+2H2O��
��Ȼ����ú��ʯ�ͺϳ�Ϊ����ʯȼ�ϣ�
��2��A��úȼ�����ɶ�����������ˮ�����������γ����꣬��������ȼú��ֱ�ӹ�ϵ��
B��úȼ�յIJ����Ƕ�����̼��������̼���γ�����ЧӦ����Ҫ���壬������ЧӦ��ȼú��ֱ�ӹ�ϵ��
C���������ƻ���Ҫ�Ƿ��������ŷ���ɵģ���ȼúû��ֱ�ӹ�ϵ��
D��ú����ȫȼ�ջ��������С��������ɿ�������������࣬�ʲ��������⣻
��ѡC
��3������ͼʾ��֪��A�����Ƕ�����������Ԫ�غ���Ԫ�ص�������Ϊ��14��16��2=7��16��
������A��B��C�ķ��Ӷ����ɲ�ͬ��ԭ�ӹ��ɵĻ�������ӣ�������D�ķ���Ϊͬ��ԭ�ӹ��ɵĵ��ʵķ��ӣ�
������A�ķ���ΪNO2���ӡ�����B�ķ���ΪCO���ӡ�����C�ķ���ΪCO2���ӡ�����D�ķ���ΪN2���ӣ���˷�Ӧ�Ļ�ѧ����ʽΪ2NO2+4CO 4CO2+N2��
4CO2+N2��
�ڸ÷�Ӧ�У�����C��D��������=��4��44����28=44��7��
�ʴ�Ϊ����1��CH4+2O2 CO2+2H2O��ʯ�ͣ���2��C����3����7��16����A��B��C����44��7��
CO2+2H2O��ʯ�ͣ���2��C����3����7��16����A��B��C����44��7��
��������1�����ݻ�ѧ����ʽ����д������ȷ��д��ѧ����ʽ����Ȼ����ú��ʯ�ͺϳ�Ϊ����ʯȼ�ϣ�
��2������úȼ�ղ�����Ⱦ�����������н��
��3�����ȸ���ͼʾ���жϳ�ABCD�Ļ�ѧʽ��ȼ�ո��ݻ�ѧʽ����Ԫ�ص������ȣ��ж��ǻ����ﻹ�ǵ��ʣ����ݻ�ѧ����ʽ���������������ȣ�
���������⿼���˻�ѧ����ʽ����д�����ݻ�ѧʽ�ļ��㣬��ʾ��ͼ�����ӹ���ģ��ͼ��ʶ���֪ʶ������ȫ�森
 CO2+2H2O��
CO2+2H2O����Ȼ����ú��ʯ�ͺϳ�Ϊ����ʯȼ�ϣ�
��2��A��úȼ�����ɶ�����������ˮ�����������γ����꣬��������ȼú��ֱ�ӹ�ϵ��
B��úȼ�յIJ����Ƕ�����̼��������̼���γ�����ЧӦ����Ҫ���壬������ЧӦ��ȼú��ֱ�ӹ�ϵ��
C���������ƻ���Ҫ�Ƿ��������ŷ���ɵģ���ȼúû��ֱ�ӹ�ϵ��
D��ú����ȫȼ�ջ��������С��������ɿ�������������࣬�ʲ��������⣻
��ѡC
��3������ͼʾ��֪��A�����Ƕ�����������Ԫ�غ���Ԫ�ص�������Ϊ��14��16��2=7��16��
������A��B��C�ķ��Ӷ����ɲ�ͬ��ԭ�ӹ��ɵĻ�������ӣ�������D�ķ���Ϊͬ��ԭ�ӹ��ɵĵ��ʵķ��ӣ�
������A�ķ���ΪNO2���ӡ�����B�ķ���ΪCO���ӡ�����C�ķ���ΪCO2���ӡ�����D�ķ���ΪN2���ӣ���˷�Ӧ�Ļ�ѧ����ʽΪ2NO2+4CO
 4CO2+N2��
4CO2+N2���ڸ÷�Ӧ�У�����C��D��������=��4��44����28=44��7��
�ʴ�Ϊ����1��CH4+2O2
 CO2+2H2O��ʯ�ͣ���2��C����3����7��16����A��B��C����44��7��
CO2+2H2O��ʯ�ͣ���2��C����3����7��16����A��B��C����44��7����������1�����ݻ�ѧ����ʽ����д������ȷ��д��ѧ����ʽ����Ȼ����ú��ʯ�ͺϳ�Ϊ����ʯȼ�ϣ�
��2������úȼ�ղ�����Ⱦ�����������н��
��3�����ȸ���ͼʾ���жϳ�ABCD�Ļ�ѧʽ��ȼ�ո��ݻ�ѧʽ����Ԫ�ص������ȣ��ж��ǻ����ﻹ�ǵ��ʣ����ݻ�ѧ����ʽ���������������ȣ�
���������⿼���˻�ѧ����ʽ����д�����ݻ�ѧʽ�ļ��㣬��ʾ��ͼ�����ӹ���ģ��ͼ��ʶ���֪ʶ������ȫ�森

��ϰ��ϵ�д�
�����Ŀ