��Ŀ����
�ҹ�Ŀǰʹ�õ�ȼ����Ҫ��ú��ʯ�ͣ������úȼ��ʱ���ɵ�SO2����Ⱦ��������1��ij�������糧ÿ��ȼ�պ���1.6%��ú100t����ú�е���ȫ��ת��ΪSO2����ó�ÿ�����SO2
��2������������ˮ���պ���γ����꣬�й������˵����ȷ����
A�������ƻ��������
B������pH��ֽ�ⶨ���������
C��pH��7����ˮΪ����
��3�����ұ��涨��ҵ������SO2�������ó���0.15mg/m3����ҵ�ϲ���SO2�ĺ��������Ը��ݷ�Ӧ��SO2+2H2O+I2�TH2SO4+2HI����ȡ�ó�����1m3����0.0254%�ĵ⣨I2����Һ2gǡ����ȫ��Ӧ������ó��ŷŵķ�����SO2�����Ƿ���Ϲ��ұ����������д�ڴ���ֽ�ϣ���
��������1���ó�ÿ�����SO2
=3?2t
��2���й������˵����ȷ���ǣ�A�������ƻ��������B������pH��ֽ�ⶨ��������ȣ���ѡAB��
��3���⣺��1 m3��ҵ �����к���SO2������Ϊx
SO2+2H2O+I2=H2SO4+2HI
64 254
X 0.0254%��2g
=
��֮�ã�X=1.28��10-4g
1m3��ҵ�����к���SO2����Ϊ0.128 mgС��0.15 mg�����Ϲ��ұ���
| 100t��1.6%��64 |
| 32 |
��2���й������˵����ȷ���ǣ�A�������ƻ��������B������pH��ֽ�ⶨ��������ȣ���ѡAB��
��3���⣺��1 m3��ҵ �����к���SO2������Ϊx
SO2+2H2O+I2=H2SO4+2HI
64 254
X 0.0254%��2g
| 64 |
| X |
| 254 |
| 0.0254X2g |
��֮�ã�X=1.28��10-4g
1m3��ҵ�����к���SO2����Ϊ0.128 mgС��0.15 mg�����Ϲ��ұ���
����⣺��1��ij�������糧ÿ��ȼ�պ���1.6%��ú100t����ú�е���ȫ��ת��ΪSO2����ó�ÿ�����SO2
=3?2t
��2���й������˵����ȷ��A�������ƻ��������B������pH��ֽ�ⶨ��������ȣ���ѡAB��
��3���⣺��1 m3��ҵ �����к���SO2������Ϊx
SO2+2H2O+I2=H2SO4+2HI
64 254
X 0.0254%��2g
=
��֮�ã�X=1.28��10-4g
1m3��ҵ�����к���SO2����Ϊ0.128 mgС��0.15 mg�����Ϲ��ұ���
�ʴ�Ϊ����1��3.2 ��2��AB ��3��X=1.28��10-4g
| 100t��1.6%��64 |
| 32 |
��2���й������˵����ȷ��A�������ƻ��������B������pH��ֽ�ⶨ��������ȣ���ѡAB��
��3���⣺��1 m3��ҵ �����к���SO2������Ϊx
SO2+2H2O+I2=H2SO4+2HI
64 254
X 0.0254%��2g
| 64 |
| X |
| 254 |
| 0.0254X2g |
��֮�ã�X=1.28��10-4g
1m3��ҵ�����к���SO2����Ϊ0.128 mgС��0.15 mg�����Ϲ��ұ���
�ʴ�Ϊ����1��3.2 ��2��AB ��3��X=1.28��10-4g
���������⿼�鳣��ȼ�ϵ�ʹ�ü�����Χ������Ӱ�죮�����úȼ��ʱ���ɵ�SO2����Ⱦ������

��ϰ��ϵ�д�
�����Ŀ
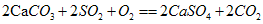 ��ʵ��֤��ʯ�ҽ�[Ca(OH)2]�ڿ�����Ҳ������SO2��������ƺ��������ʣ�д���÷�Ӧ�Ļ�ѧ����ʽ��________________________________��
��ʵ��֤��ʯ�ҽ�[Ca(OH)2]�ڿ�����Ҳ������SO2��������ƺ��������ʣ�д���÷�Ӧ�Ļ�ѧ����ʽ��________________________________��