��Ŀ����
ע������һ����ͨ��ҽ����е�������ڻ�ѧʵ���о������֣�����ijЩʵ������Ĺ۲��ʵ����̵ĸĽ��������벻����Ч����
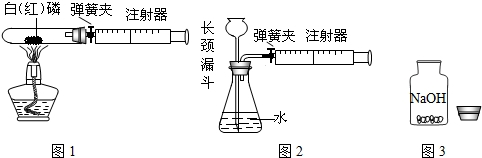
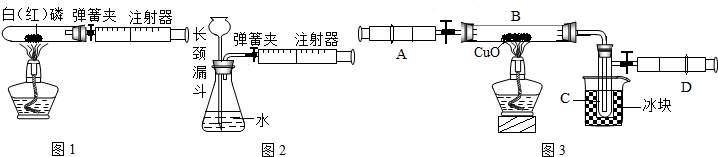
��1��ͼ1��50 mL�Թ�����Ӧ��������ȼ�վ����ܱ���������У��ɷ�ֹ������Ⱦ��������50mLע�������������ȴ���20 mL�̶ȴ���������ȼ�����ĵ����������
�������ټ��װ�õ������ԣ���װҩƷ�������������ۼн����ɼУ����Ȱ��ף��۲��Թ�������������Ϊ______����ȼ�ս������Թ���ȴ����ɼУ����Կ��������������Ƶ�Լ______mL�̶ȴ���ȡ����ֵ����˵���������������������ԼΪ______��
��2��ͼ2������ע���������ķ������Լ��װ�õ������ԣ�������������������ʱ�����װ�����������ã����ܹ۲쵽______������ţ�
A��ע��������Һ�� B��ƿ��Һ������
C������©����Һ������ D������©���¶˹ܿڲ�������
��3����ȤС���߽�ʵ���ҿ�����һ������г�ġ�����������ͼ3��������˾��������뵽��ȡ�ø�ҩƷʱ�Լ�ƿƿ��Ӧ______�������ϣ�ȡ�ú�Ӧ��������ƿ���ܷⱣ�棬������Ϊ______��______��
��4������ȤС��ͬѧΪ�˲ⶨij���ֱ��ʵ��ռ���Ʒ��Na2CO3�������������ֽ���������ʵ�飺
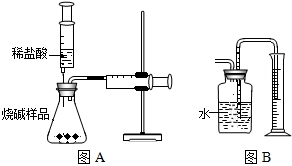
���������������ͼA��ʾ��װ�ã�ͨ����Ӧ���Ҳ�ע�������ռ���������������Na2CO3��������������֪��״̬��CO2���ܶȣ���ʹ��ע�����������Ϊ20mL����д����ƿ���������仯�Ļ�ѧ����ʽ��______��
______��С����Ϊ�÷�������ȷ���Na2CO3������������������Ϊ______��С����Ϊ�÷�������Ʒ��ȡ����Ҳ��Ҫһ���Ŀ��ƣ�������Ϊ______��
����ͬѧ�����ͼB����ͼA�е��ռ�װ�ã�������CO2��������______���ƫ����ƫС���������䡱����������______����ĸĽ�������______�����ƿ��ԭ�еĿ�����ʵ����______����С���û�С���Ӱ�죮

��1��ͼ1��50 mL�Թ�����Ӧ��������ȼ�վ����ܱ���������У��ɷ�ֹ������Ⱦ��������50mLע�������������ȴ���20 mL�̶ȴ���������ȼ�����ĵ����������
�������ټ��װ�õ������ԣ���װҩƷ�������������ۼн����ɼУ����Ȱ��ף��۲��Թ�������������Ϊ______����ȼ�ս������Թ���ȴ����ɼУ����Կ��������������Ƶ�Լ______mL�̶ȴ���ȡ����ֵ����˵���������������������ԼΪ______��
��2��ͼ2������ע���������ķ������Լ��װ�õ������ԣ�������������������ʱ�����װ�����������ã����ܹ۲쵽______������ţ�
A��ע��������Һ�� B��ƿ��Һ������
C������©����Һ������ D������©���¶˹ܿڲ�������
��3����ȤС���߽�ʵ���ҿ�����һ������г�ġ�����������ͼ3��������˾��������뵽��ȡ�ø�ҩƷʱ�Լ�ƿƿ��Ӧ______�������ϣ�ȡ�ú�Ӧ��������ƿ���ܷⱣ�棬������Ϊ______��______��

��4������ȤС��ͬѧΪ�˲ⶨij���ֱ��ʵ��ռ���Ʒ��Na2CO3�������������ֽ���������ʵ�飺
���������������ͼA��ʾ��װ�ã�ͨ����Ӧ���Ҳ�ע�������ռ���������������Na2CO3��������������֪��״̬��CO2���ܶȣ���ʹ��ע�����������Ϊ20mL����д����ƿ���������仯�Ļ�ѧ����ʽ��______��
______��С����Ϊ�÷�������ȷ���Na2CO3������������������Ϊ______��С����Ϊ�÷�������Ʒ��ȡ����Ҳ��Ҫһ���Ŀ��ƣ�������Ϊ______��
����ͬѧ�����ͼB����ͼA�е��ռ�װ�ã�������CO2��������______���ƫ����ƫС���������䡱����������______����ĸĽ�������______�����ƿ��ԭ�еĿ�����ʵ����______����С���û�С���Ӱ�죮
��1�����Ȱ��ף��۲��Թ��������������ǣ�����ȼ�գ���������������ռ�������������Ϊ20%������ע��������Ӧ�����ƶ���10mL����
��2��ע������������������ƿ������ѹǿ��С��������ѹ�������س���©��ѹ����ƿ�У��ڳ���©���¶˻ῴ��������ð������ѡD��
��3��ȡ��ҩƷʱ���Լ�ƿ��Ӧ���ţ�����������Ҫ�ܷ���ﱣ�棬��Ϊ���������׳��⡢�������տ����ж�����̼�����ʣ�
��4����ƿ���������仯�Ļ�ѧ����ʽΪ��HCl+NaOH�TNaCl+H2O��2HCl+Na2CO3�TCO2��+2NaCl��С����Ϊ�÷�������ȷ���Na2CO3������������������Ϊ�����Ķ�����ֻ̼�в��ֽ���ע�����У����в�������ƿ�У���Ϊע�������ݻ���С��������Ʒ��ȡ����Ҳ��Ҫһ���Ŀ��ƣ�ͼBʹ�õ�����ˮ���ռ�������̼����Ϊ������̼������ˮ���������ռ�������̼����ƫС������̼�����Ʊ�����Һ����ˮ�ռ�������̼����Ϊ���õ�����ˮ�����������Թ��ƿ�еĿ�����ʵ����û��Ӱ�죮
�ʴ�Ϊ����1�����̰���ȼ�գ������������̣�10��20%��
��2��D��
��3���������������׳��⡢�����տ����ж�����̼�����ʣ�
��4��HCl+NaOH�TNaCl+H2O��2HCl+Na2CO3�TCO2��+2NaCl�������Ķ�����ֻ̼�в��ֽ���ע�����У����в�������ƿ�У�ע���������ռ�������̼���������ȵģ�ƫС��������̼������ˮ����̼�����Ʊ�����Һ����ˮ��û�У�
��2��ע������������������ƿ������ѹǿ��С��������ѹ�������س���©��ѹ����ƿ�У��ڳ���©���¶˻ῴ��������ð������ѡD��
��3��ȡ��ҩƷʱ���Լ�ƿ��Ӧ���ţ�����������Ҫ�ܷ���ﱣ�棬��Ϊ���������׳��⡢�������տ����ж�����̼�����ʣ�
��4����ƿ���������仯�Ļ�ѧ����ʽΪ��HCl+NaOH�TNaCl+H2O��2HCl+Na2CO3�TCO2��+2NaCl��С����Ϊ�÷�������ȷ���Na2CO3������������������Ϊ�����Ķ�����ֻ̼�в��ֽ���ע�����У����в�������ƿ�У���Ϊע�������ݻ���С��������Ʒ��ȡ����Ҳ��Ҫһ���Ŀ��ƣ�ͼBʹ�õ�����ˮ���ռ�������̼����Ϊ������̼������ˮ���������ռ�������̼����ƫС������̼�����Ʊ�����Һ����ˮ�ռ�������̼����Ϊ���õ�����ˮ�����������Թ��ƿ�еĿ�����ʵ����û��Ӱ�죮
�ʴ�Ϊ����1�����̰���ȼ�գ������������̣�10��20%��
��2��D��
��3���������������׳��⡢�����տ����ж�����̼�����ʣ�
��4��HCl+NaOH�TNaCl+H2O��2HCl+Na2CO3�TCO2��+2NaCl�������Ķ�����ֻ̼�в��ֽ���ע�����У����в�������ƿ�У�ע���������ռ�������̼���������ȵģ�ƫС��������̼������ˮ����̼�����Ʊ�����Һ����ˮ��û�У�

��ϰ��ϵ�д�
 ������������ϵ�д�
������������ϵ�д�
�����Ŀ
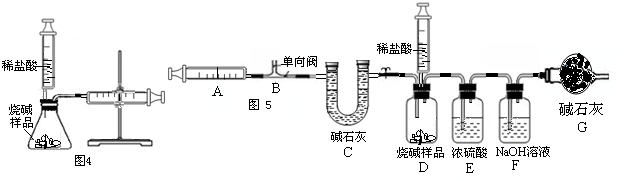


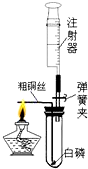 ע������һ����ͨ��ҽ����е�������ڻ�ѧʵ���з�����Խ��Խ������ã�ij��ѧ��ȤС��Ϊ�˲ⶨ�����������ĺ���������������̽�����
ע������һ����ͨ��ҽ����е�������ڻ�ѧʵ���з�����Խ��Խ������ã�ij��ѧ��ȤС��Ϊ�˲ⶨ�����������ĺ���������������̽�����