��Ŀ����
��ͼ��ˮ������ˮ���е�ˮ��������ˮ����Ҫ���̣�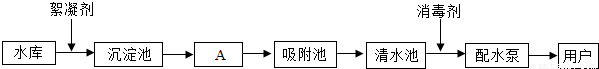
��1��ˮ���е�ˮ����______����������������
��2����A����ͨ��______����������ƣ���ȥˮ�еĹ������ʣ�
�ڶ������ȣ�ClO2����һ�ָ�Ч����������������Ԫ�صĻ��ϼ�Ϊ______�����������������ƣ�NaClO2����Ӧ��ȡClO2�ķ�Ӧ����ʽΪ��Cl2+2NaClO2�T2ClO2+2X����x�Ļ�ѧʽ��______
�۹��ҹ涨������ˮPH��ΧΪ6.5��8.5��Ϊ�˲ⶨ��������ˮ�Ƿ�ﵽ��һ��������______�����м�⣮
��3������ˮ��ͨ��������Cl-��������ˮ���еμ�����ϡ�����______���飬������______����˵��ˮ�к���Cl-��ˮ�е�Ca2+��Mg2+���Ⱥ�ת��Ϊ��������ͨ����˵��ˮ����ˮ������Ҫ�ɷ�Ϊ______��
��4��������ˮ��һ������Ϊ����ˮ��ʵ�鷽����______��ʵ������ȡ����ˮ��������ƿ�ͨ��Ҫ���뼸����ʯ�����Ƭ��������______��
���𰸡���������1������ˮ���е���ɷ������
��2���ٸ��ݹ��˵�ԭ�������ش�
�ڸ��ݻ��ϼ�ԭ�������غ㶨�ɷ����ش�
������PH��ֽ�ⶨ��Һ�����ȣ�
��3�����������ӵļ��鷽����ˮ���ijɷַ����ش�
��4�����������ԭ����ע������������
����⣺��1������ˮ���е�ˮ���ڳ����Ļ����У�������һЩ���ʣ����ԣ�ˮ���е�ˮ���ڻ���
��2���٣�A����ͨ�����˳�ȥˮ�еĹ������ʣ�
���ڶ������ȣ�ClO2���У�����-2�ۣ����ȵĻ��ϼ���+4�ۣ���Cl2+2NaClO2�T2ClO2+2X����ʽ������У���ԭ�ӡ���ԭ�ӡ���ԭ�ӵ���Ŀ�ֱ���4��2��2�����ұ�����ԭ�ӡ���ԭ�ӵ���Ŀ�ֱ���2��2���������غ㶨�ɷ�Ӧǰ��ԭ�ӵ����༰��Ŀ�����֪��X�Ļ�ѧʽ��NaCl��
��Ϊ�˲ⶨ��������ˮ�����ȣ�Ӧ��PH��ֽ�����м�⣻
��3������ˮ��ͨ��������Cl-��������ˮ���еμ�����ϡ��������������飬�����ְ�ɫ��������˵��ˮ�к���Cl-��ˮ�е�Ca2+��Mg2+���Ⱥ�ת��Ϊ��������ͨ����˵��ˮ����ˮ������Ҫ�ɷ�Ϊ̼��ƺ�������þ��
��4��������ˮ��һ������Ϊ����ˮ��ʵ�鷽��������ʵ������ȡ����ˮ��������ƿ�ͨ��Ҫ���뼸����ʯ�����Ƭ�������Ƿ�ֹ���У�
�ʴ�Ϊ����1��������2���ٹ��ˣ���+4��NaCl����PH��ֽ����3����������̼��ƺ�������þ����4������ֹ���У�
���������⿼����ѧ����ˮ�ľ��������ϼ�ԭ�������غ㶨�ɣ����ȵIJⶨ��֪ʶ�����պ�Ӧ�ã��ѶȲ����������֪ʶ����˳�����
��2���ٸ��ݹ��˵�ԭ�������ش�
�ڸ��ݻ��ϼ�ԭ�������غ㶨�ɷ����ش�
������PH��ֽ�ⶨ��Һ�����ȣ�
��3�����������ӵļ��鷽����ˮ���ijɷַ����ش�
��4�����������ԭ����ע������������
����⣺��1������ˮ���е�ˮ���ڳ����Ļ����У�������һЩ���ʣ����ԣ�ˮ���е�ˮ���ڻ���
��2���٣�A����ͨ�����˳�ȥˮ�еĹ������ʣ�
���ڶ������ȣ�ClO2���У�����-2�ۣ����ȵĻ��ϼ���+4�ۣ���Cl2+2NaClO2�T2ClO2+2X����ʽ������У���ԭ�ӡ���ԭ�ӡ���ԭ�ӵ���Ŀ�ֱ���4��2��2�����ұ�����ԭ�ӡ���ԭ�ӵ���Ŀ�ֱ���2��2���������غ㶨�ɷ�Ӧǰ��ԭ�ӵ����༰��Ŀ�����֪��X�Ļ�ѧʽ��NaCl��
��Ϊ�˲ⶨ��������ˮ�����ȣ�Ӧ��PH��ֽ�����м�⣻
��3������ˮ��ͨ��������Cl-��������ˮ���еμ�����ϡ��������������飬�����ְ�ɫ��������˵��ˮ�к���Cl-��ˮ�е�Ca2+��Mg2+���Ⱥ�ת��Ϊ��������ͨ����˵��ˮ����ˮ������Ҫ�ɷ�Ϊ̼��ƺ�������þ��
��4��������ˮ��һ������Ϊ����ˮ��ʵ�鷽��������ʵ������ȡ����ˮ��������ƿ�ͨ��Ҫ���뼸����ʯ�����Ƭ�������Ƿ�ֹ���У�
�ʴ�Ϊ����1��������2���ٹ��ˣ���+4��NaCl����PH��ֽ����3����������̼��ƺ�������þ����4������ֹ���У�
���������⿼����ѧ����ˮ�ľ��������ϼ�ԭ�������غ㶨�ɣ����ȵIJⶨ��֪ʶ�����պ�Ӧ�ã��ѶȲ����������֪ʶ����˳�����

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
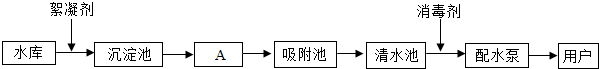


 ��ͼ��ˮ������ˮ���е�ˮ��������ˮ����Ҫ���̣�
��ͼ��ˮ������ˮ���е�ˮ��������ˮ����Ҫ���̣�