��Ŀ����
С�մ�θ��ƽ����ϲ���dz��õ��к�θ���ҩ���1��С�մ�ÿƬ��0.50gNaHCO3��2ƬС�մ��θ�ᷴӦ�����к͵�HCl��������______��
��2��θ��ƽÿƬ0.245gAl��OH��3���к�θ��ʱ��6ƬС�մ�Ƭ�൱��θ��ƽ______Ƭ��
��3�����ʡ���ϲ���Ļ�ѧʽΪAl2Mg6��OH��16CO3?4H2O����ȡ������30.1g������20%������ʹ���ܽ⣬����������______gǡ����ȫ��Ӧ������______gCO2��
���𰸡���������1������̼�����Ƶ��������Ȼ��������
��2�����ݷ���ʽ�ҳ�����������̼������֮��Ĺ�ϵ
��3�����ݻ�ѧ����ʽ����֪Al2Mg6��OH��16CO3?4H2O���������������������Ͷ�����̼��������
����⣺��1���豻�к͵�HCl������Ϊx
NaHCO3+HCl�TNaCl+H2O+CO2��
84 36.5
0.5g×2 x
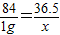
x=0.438g
��2�����൱������������Ƭ��Ϊy
�ɷ���ʽAl��OH��3+3HCl=AlCl3+3H2O��NaHCO3+HCl�TNaCl+H2O+CO2��
�ù�ϵʽAl��OH��3��3HCl��3NaHCO3
78 3×84
0.245g×y 0.5g×6

y=3.8Ƭ
��3����������������Ϊz��ͬʱ���ɶ�����̼������Ϊm
Al2Mg6��OH��16CO3?4H2O+18HCl=2AlCl3+6MgCl2+21H2O+CO2��
602 18×36.5 44
30.1g x×20% m

x=164.25g
m=2.2g
�ʴ�Ϊ����1��0.438g��2��3.8��3��164.25g 2.2
���������⿼�黯ѧ����ʽ�Ļ������㣬������֪���������ؼ���ȷд����ѧ����ʽ���ҳ���֪��ʹ����
��2�����ݷ���ʽ�ҳ�����������̼������֮��Ĺ�ϵ
��3�����ݻ�ѧ����ʽ����֪Al2Mg6��OH��16CO3?4H2O���������������������Ͷ�����̼��������
����⣺��1���豻�к͵�HCl������Ϊx
NaHCO3+HCl�TNaCl+H2O+CO2��
84 36.5
0.5g×2 x
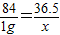
x=0.438g
��2�����൱������������Ƭ��Ϊy
�ɷ���ʽAl��OH��3+3HCl=AlCl3+3H2O��NaHCO3+HCl�TNaCl+H2O+CO2��
�ù�ϵʽAl��OH��3��3HCl��3NaHCO3
78 3×84
0.245g×y 0.5g×6

y=3.8Ƭ
��3����������������Ϊz��ͬʱ���ɶ�����̼������Ϊm
Al2Mg6��OH��16CO3?4H2O+18HCl=2AlCl3+6MgCl2+21H2O+CO2��
602 18×36.5 44
30.1g x×20% m

x=164.25g
m=2.2g
�ʴ�Ϊ����1��0.438g��2��3.8��3��164.25g 2.2
���������⿼�黯ѧ����ʽ�Ļ������㣬������֪���������ؼ���ȷд����ѧ����ʽ���ҳ���֪��ʹ����

��ϰ��ϵ�д�
 Ʒѧ˫�ž�ϵ�д�
Ʒѧ˫�ž�ϵ�д� Сѧ��ĩ���100��ϵ�д�
Сѧ��ĩ���100��ϵ�д� ��ĩ��ϰ���ϵ�д�
��ĩ��ϰ���ϵ�д� ����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
�����Ŀ