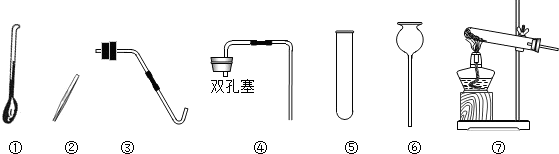
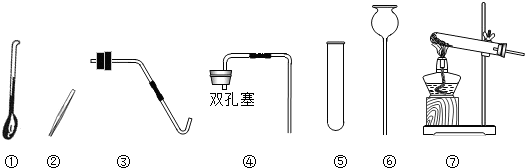
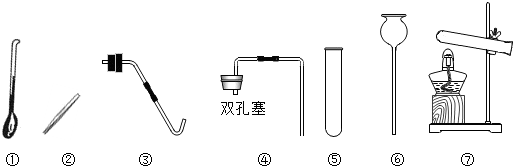
��Ŀ����
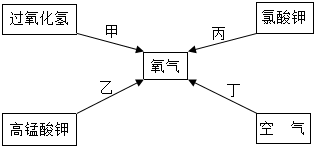
����������������ش��������⣺
��1�������ݵ�������
��2������ü��ȸ��������ȡ��������ȡװ�����ѡ������
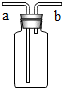
��3���ڢ����ռ���������̼��������ʢ��ˮ���ձ��У�һ��ʱ�����Һ��

��1�������ݵ�������
�Թ�
�Թ�
��ȡ�÷�ĩ״��������õ�����������
��
������ţ�����2������ü��ȸ��������ȡ��������ȡװ�����ѡ������
��
��
������ţ����仯ѧ����ʽΪ��2KMnO4
K2MnO4+MnO2+O2��
| ||
2KMnO4
K2MnO4+MnO2+O2��
���ռ������ķ���Ϊ
| ||
��ˮ���������ſ���
��ˮ���������ſ���
������3���ڢ����ռ���������̼��������ʢ��ˮ���ձ��У�һ��ʱ�����Һ��
����
����
������������䡱����������������̼������ˮ���Թ��ڵ�������٣�ѹǿ��С��Ե��
������̼������ˮ���Թ��ڵ�������٣�ѹǿ��С��Ե��
����������1����Ϥ�����������˽����ƣ�ȡ�÷�ĩ״��������õ�ҩ�ף�
��2�������ø��������ȡ������Ҫ���ȣ�ѡ����װ�ã����ݷ�Ӧ���Ӧ������������д����ѧ����ʽ�������������ܶȺ���ˮ��ѡ���ռ�װ�ã�
��3�����ݶ�����̼�ܹ�����ˮ������
��2�������ø��������ȡ������Ҫ���ȣ�ѡ����װ�ã����ݷ�Ӧ���Ӧ������������д����ѧ����ʽ�������������ܶȺ���ˮ��ѡ���ռ�װ�ã�
��3�����ݶ�����̼�ܹ�����ˮ������
����⣺��1��ͼ�Т����Թܣ�ȡ�÷�ĩ״��������õ�ҩ�ף�
�ʴ�Ϊ���Թܣ��٣�
��2���ø��������ȡ������Ҫ���ȣ���ȡװ��Ӧ���âߣ���Ӧ����ʽ�ǣ�2KMnO4
K2MnO4+MnO2+O2�����������ܶȴ��ڿ������ܶȣ���������ˮ����������ˮ���������ſ������ռ���
�ʴ�Ϊ���ߣ�2KMnO4
K2MnO4+MnO2+O2������ˮ���������ſ�����
��3��������̼������ˮ���Թ��ڵ�������٣�ѹǿ��С��������������ѹǿ�������¢���Һ��������
�ʴ�Ϊ��������������̼������ˮ���Թ��ڵ�������٣�ѹǿ��С��Ե�ʣ�
�ʴ�Ϊ���Թܣ��٣�
��2���ø��������ȡ������Ҫ���ȣ���ȡװ��Ӧ���âߣ���Ӧ����ʽ�ǣ�2KMnO4
| ||
�ʴ�Ϊ���ߣ�2KMnO4
| ||
��3��������̼������ˮ���Թ��ڵ�������٣�ѹǿ��С��������������ѹǿ�������¢���Һ��������
�ʴ�Ϊ��������������̼������ˮ���Թ��ڵ�������٣�ѹǿ��С��Ե�ʣ�
������ʵ�鷢��װ�õ�ѡ��Ҫ���ݷ�Ӧ���״̬�ͷ�Ӧ�������ռ�װ�õ�ѡ��Ҫ����������ܶȺ���ˮ�ԣ�

��ϰ��ϵ�д�
 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д� ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�
�����Ŀ