��Ŀ����
��һƿ���壬������CO��CO2��H2O��H2�е�һ�ֻ�����ɡ�Ϊ�˷���������ijɷ֣�ȡ�������������ʵ�飬ʵ�鷽��������£�
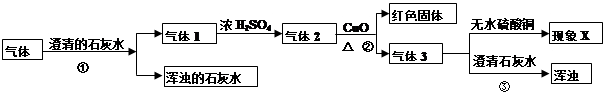
���������ϣ���ɫ��ˮ����ͭ��ĩ��ˮ����ɫ��ŨH2SO4����ˮ�ԣ��ܸ���ijЩ���塣��
��1���������ܴ��ڵ������У�����Ѫ�쵰��ϵ�������______��
��2��ʵ�������û����ŨH2SO4��������1���������������Ӱ��______��
��3��������XΪ����ˮ����ͭ����ɫ���������������� ��������XΪ����ˮ����ͭ����ɫ���������������� ��
��4������һ�������ķ�Ӧ����ʽ�� ��
��5���٢۷����ķ�Ӧ��ͬ���䷴Ӧ�ķ���ʽ�� ��
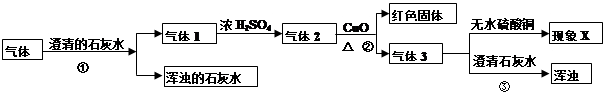
���������ϣ���ɫ��ˮ����ͭ��ĩ��ˮ����ɫ��ŨH2SO4����ˮ�ԣ��ܸ���ijЩ���塣��
��1���������ܴ��ڵ������У�����Ѫ�쵰��ϵ�������______��
��2��ʵ�������û����ŨH2SO4��������1���������������Ӱ��______��
��3��������XΪ����ˮ����ͭ����ɫ���������������� ��������XΪ����ˮ����ͭ����ɫ���������������� ��
��4������һ�������ķ�Ӧ����ʽ�� ��
��5���٢۷����ķ�Ӧ��ͬ���䷴Ӧ�ķ���ʽ�� ��
��1��CO ��2����Ӱ��
(3)һ������H2��һ������H2��4��CuO + CO Cu + CO2
Cu + CO2
(5)CO2 + Ca(OH)2 ="=" CaCO3�� + H2O
(3)һ������H2��һ������H2��4��CuO + CO
 Cu + CO2
Cu + CO2 (5)CO2 + Ca(OH)2 ="=" CaCO3�� + H2O
������������ݼ�����������ʷ�����
��1��һ����̼�ж���������ѪҺ�е�Ѫ�쵰��ϣ��Ӷ�����������֯��������ȱ����������
��2����Ӱ�죬���û����ŨH2SO4��������1����ô����2��3�ж��Ậ��ˮ��������Xһ���ǡ���ˮ����ͭ����ɫ����������˵����ˮ��ʵ������д����ˮ����������������ͭ��Ӧ���ɵ�ˮ��Ҳ����֤�������Ĵ��ڡ�
��3����������1ͨ����Ũ���ᣬ������2�п϶�û��ˮ��������X�С���ˮ����ͭ����ɫ����˵������3�к���ˮ����˵����CuO��Ӧ�Ļ�������п϶��к���������������X�С���ˮ����ͭ����ɫ����˵������3�в�����ˮ������CuO��Ӧ�Ļ�������п϶�������������
��4����������3ͨ������ʯ��ˮ�������ʯ��ˮ����ǡ���˵������3��һ������CO2����֮ǰ��CO2�ѱ���ȥ������˵����CuO��Ӧ�Ļ�������п϶���CO���ʷ�Ӧ����һ�������ķ�Ӧ����ʽ��CuO+CO
 Cu+CO2��
Cu+CO2����5����ʹ����ʯ��ˮ����ǵ�ԭ���Ƕ�����̼������������Һ������Ӧ���ɲ�����ˮ��̼��Ƴ��������䷴Ӧ����ʽΪCO2 +Ca(OH)2=CaCO3��+H2O��
�����������Ĺؼ��ǣ���dz�������ļ�������ӷ������������ݣ��Լ�������������������̼��һ����̼�������������ʡ�

��ϰ��ϵ�д�
�����Ŀ