��Ŀ����
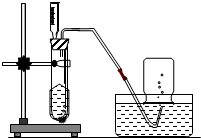 ʵ������H2O2 ��Һ��MnO2����������ȡO2���ش��������⣬
ʵ������H2O2 ��Һ��MnO2����������ȡO2���ش��������⣬��1��С���������ͼ��ʾ����ȡװ�ã�������Ӧ�Ļ�ѧ����ʽ�ǣ�
2H2O2
2H2O+O2��
| ||
2H2O2
2H2O+O2��
��װ������ע�������泤��©�����ŵ���
| ||
����ʱ���Ʒ�Ӧ�ķ�����ֹͣ
����ʱ���Ʒ�Ӧ�ķ�����ֹͣ
������һ�����ɣ���2��ͬ���С����ӷ�Ӧ�Ļ�����з����MnO2���壬����Ҫ������
����
����
���˲����б����õ��IJ����������ձ���©����������
�ձ���©����������
����3����һ���СԪ�ü��ȸ�����صķ�����ȡ500ml������������СԪ������Ҫ������ض��ٿˣ�����֪�������ܶ�Ϊ1.43g/L ��
��������1�����ݹ�������ֽ�ķ�Ӧԭ�������ע�������泤��©������ʱ���Ʒ�Ӧ�ķ�����ֹͣ��������㣻
��2���������̲�����ˮ�����ù��˷����з��룬����ʱ�õ����������ձ���©����������������̨��
��3�����ݸ�������������Ļ�ѧ����ʽ���
��2���������̲�����ˮ�����ù��˷����з��룬����ʱ�õ����������ձ���©����������������̨��
��3�����ݸ�������������Ļ�ѧ����ʽ���
����⣺��1�����������ڶ�������������������������������ˮ����ע�������泤��©������ʱ���Ʒ�Ӧ�ķ�����ֹͣ��������㣻
�ʴ�Ϊ��2H2O2
2H2O+O2��������ʱ���Ʒ�Ӧ�ķ�����ֹͣ��
��2�������ǰѲ�����Һ��Ĺ������ʸ�Һ����뿪����һ�ֻ�������ķ������������̲�����ˮ���ʿ��ù��˷����з��룬����ʱ�õ����������ձ���©����������������̨��
�ʴ�Ϊ�����ˣ��ձ���©������������
��3����ȡ������������1.43g/L��0.5L=0.72g
��СԪ������Ҫ������ص�����ΪX
2KMnO4
K2MnO4+MnO2+O2��
316 32
X 0.72g
=
X=7.11g
��СԪ������Ҫ�������7.11�ˣ�
�ʴ�Ϊ��2H2O2
| ||
��2�������ǰѲ�����Һ��Ĺ������ʸ�Һ����뿪����һ�ֻ�������ķ������������̲�����ˮ���ʿ��ù��˷����з��룬����ʱ�õ����������ձ���©����������������̨��
�ʴ�Ϊ�����ˣ��ձ���©������������
��3����ȡ������������1.43g/L��0.5L=0.72g
��СԪ������Ҫ������ص�����ΪX
2KMnO4
| ||
316 32
X 0.72g
| 316 |
| X |
| 32 |
| 0.72g |
X=7.11g
��СԪ������Ҫ�������7.11�ˣ�
������������Ҫ������ʵ�������ù������⼰�������ȡ�����ķ�Ӧԭ��������ʽ�ļ��㣬���˵����֪ʶ��Ҫϸ�Ľ��

��ϰ��ϵ�д�
�����Ŀ

 ��������ˮ���ܶȱȿ����������ڿ����в�������Ӧ��
��������ˮ���ܶȱȿ����������ڿ����в�������Ӧ��
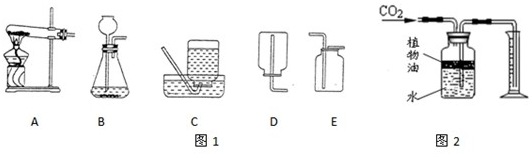

 ��������ˮ���ܶȱȿ����������ڿ����в�������Ӧ��
��������ˮ���ܶȱȿ����������ڿ����в�������Ӧ�� ��ʾΪʵ�����г����������Ʊ����ռ�װ�á�
��ʾΪʵ�����г����������Ʊ����ռ�װ�á�
 ��
�� װ��ˮ���ٽ������ ���a����b������ͨ�롣
װ��ˮ���ٽ������ ���a����b������ͨ�롣