��Ŀ����
�����ڹ�ũҵ�������ճ����������ź���Ҫ�����ã�
��1�����������________��������������������
��2������Ʒ���кܺõĿ���ʴ���ܣ���������������������ѳ�����ͭ���뽫�����ں����ϵ���ȷ����д�ڴ���ֽ�ϣ�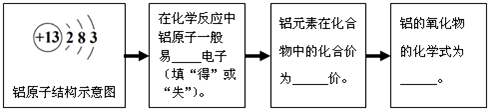
��3��ijͬѧʵ�����ʱ��Ϊ�˻��պ���CuSO4��ZnSO4��FeSO4��Һ�е��йؽ������Σ����������ʵ�鷽����
�Իش�
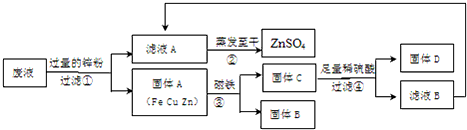
�������п�۱��������ԭ����________��
��д�����������һ��Ӧ�Ļ�ѧ����ʽ________��
��Ҫ���鲽����м����ϡ�����Ƿ������ķ���________��
������ʵ������е�������ʧ���Ժ��ԣ�������������п������________�����������������=����ԭ��Һ������п��������Ҫ����÷�Һ������ͭ����������Ҫ����________��������
�⣺��1������������ĺϽ𣬲�����г������⣬����C��Cr��Ni�ȣ����ڻ���
�ʴ�Ϊ�������
��2������ԭ�ӵĽṹʾ��ͼ���������������3�����ӣ�����ʧȥ����ʾ+3�ۣ�
�ʴ�Ϊ��ʧ��+3��Al2O3
��3������Ϊֻ�м��������п�Ż����Һ�е�����ͭ�û��ĸ�����һЩ��
�ʴ�Ϊ������Һ�е�Cu2+��Fe2+ȫ���û�����
���ڴ˹����з�����п������ͭ����������ͭ�ķ�Ӧ��
�ʴ�Ϊ��Zn+CuSO4=ZnSO4+Cu����Fe+CuSO4�TCu+FeSO4��
������е����Ƿ�������Ҫ������D���Ƿ���δ��Ӧ��п�������������û������������
�ʴ�Ϊ��ȡ����ܵ��������Թ��У���������ϡ���ᣬ�������ݲ�����������������������ݲ�����������������
����Ϊ��Ӧ����������п�ļ����ʹ��õ�������п��������ˣ��ɹ���֪ͭ����Դֻ������ͭ����������ͭ�����������õõ�ͭ��������
�ʴ�Ϊ����������D����ͭ��
��������1�����ݲ���ֵijɷֽ�ϴ�����������ĸ�����з����жϣ�
��2����Ԫ�ص�ԭ�ӽṹʾ��ͼ������֪Ԫ�صĻ�ѧ���ʺͻ��ϼۣ���Ҫ�����������Ӿ����ģ�
��3������������п�����Һ�е�����ͭ�û��ĸ�����һЩ��
����һ�лᷢ����������������Һ�ķ�Ӧ��
������е����Ƿ�������Ҫ������D���Ƿ���δ��Ӧ��п��
���õ�������п������Ӧ�ñȷ�Һ�е�����п��������Ϊ��Ӧ����������п�ļ����ʹ����������ˣ�Ҫ��������ͭ��������ͨ�����õ���ͭ��������ɣ�
�����������ѶȲ������մ�����ͻ���������Ͷ����ʷ���֪ʶ�Ŀ��飬����ʱֻҪץס��Ӧ�����н���������Һ�ķ�Ӧʵ�ʣ��������ص�ʵ����̾���˳�����⣮
�ʴ�Ϊ�������
��2������ԭ�ӵĽṹʾ��ͼ���������������3�����ӣ�����ʧȥ����ʾ+3�ۣ�
�ʴ�Ϊ��ʧ��+3��Al2O3
��3������Ϊֻ�м��������п�Ż����Һ�е�����ͭ�û��ĸ�����һЩ��
�ʴ�Ϊ������Һ�е�Cu2+��Fe2+ȫ���û�����
���ڴ˹����з�����п������ͭ����������ͭ�ķ�Ӧ��
�ʴ�Ϊ��Zn+CuSO4=ZnSO4+Cu����Fe+CuSO4�TCu+FeSO4��
������е����Ƿ�������Ҫ������D���Ƿ���δ��Ӧ��п�������������û������������
�ʴ�Ϊ��ȡ����ܵ��������Թ��У���������ϡ���ᣬ�������ݲ�����������������������ݲ�����������������
����Ϊ��Ӧ����������п�ļ����ʹ��õ�������п��������ˣ��ɹ���֪ͭ����Դֻ������ͭ����������ͭ�����������õõ�ͭ��������
�ʴ�Ϊ����������D����ͭ��
��������1�����ݲ���ֵijɷֽ�ϴ�����������ĸ�����з����жϣ�
��2����Ԫ�ص�ԭ�ӽṹʾ��ͼ������֪Ԫ�صĻ�ѧ���ʺͻ��ϼۣ���Ҫ�����������Ӿ����ģ�
��3������������п�����Һ�е�����ͭ�û��ĸ�����һЩ��
����һ�лᷢ����������������Һ�ķ�Ӧ��
������е����Ƿ�������Ҫ������D���Ƿ���δ��Ӧ��п��
���õ�������п������Ӧ�ñȷ�Һ�е�����п��������Ϊ��Ӧ����������п�ļ����ʹ����������ˣ�Ҫ��������ͭ��������ͨ�����õ���ͭ��������ɣ�
�����������ѶȲ������մ�����ͻ���������Ͷ����ʷ���֪ʶ�Ŀ��飬����ʱֻҪץס��Ӧ�����н���������Һ�ķ�Ӧʵ�ʣ��������ص�ʵ����̾���˳�����⣮

��ϰ��ϵ�д�
 �ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�����Ŀ
���ϣ�����2002�ꣴ��30�ա��й���������������ij�������ŷŵ���ˮ��ʹij��һ����Լ50����ĺ�ˮ�ܵ�������Ⱦ���Թ�ũҵ����������������������Σ�������������Ųⶨ������Ⱦ�ĺ�ˮpH�ڣ�����֮�䣬����ˮ��ָ��Ҳ���س��ꡣ
���ϣ���2002��6��1�գ����һ��������ַܾ����ġ��ر�ˮ���������������Եر�ˮ����������ȷ�涨������ָ�����£���pH�⣬����Ŀ�ĵ�λ��mg/L����
| ��������ˮ�ʷ��� ��Ŀ���� | ���� | ���� | ���� | ���� | ���� |
| �ף��У��� | 0.02 | 0.1 | 0.2 | 0.3 | 0.4 |
| �������� | 0.2 | 0.5 | 1.0 | 1.5 | 2.0 |
| ���n���� | 0.05 | 1.0 | 1.0 | 2.0 | 2.0 |
| Ǧ����b���� | 0.01 | 0.01 | 0.05 | 0.05 | 0.1 |
| pH | 6-9 | 6-9 | 6-9 | 6-9 | 6-9 |
���´ɺ��մ����߱���������к��м����ģ�b����d���ͣ�b ���ж��������࣬Ϊ��ֹ�ж������ܳ���ʢ�����������еģ�����������
�������͡������������¡�ʳ�Ρ������������á�ʳ�ס������������ġ�����