��Ŀ����
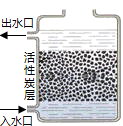 2003��6��5�����绷���յ�����Ϊ����ˮ-20��������֮��ϵ����������������й���������ۣ�
2003��6��5�����绷���յ�����Ϊ����ˮ-20��������֮��ϵ����������������й���������ۣ�
��1����ͼ���г��ϳ��۵�һ�ֻ���̿��ˮ����ʾ��ͼ�����־�ˮ�����Գ�ȥˮ�е����������ʣ�ͬʱ���û���̿��________���ó�ȥ��ζ��һЩ�ܽ�����ʣ�
��2����ʵ������Ҫ��ȥ��Ȼˮ�еĹ������ʣ����Բ���________�ķ�����
��3����������Ӳˮ�����彡��������Ҫ����ˮ��Ӳ�ȣ��ڼ�ͥ��ͨ������________�ķ�����
�⣺��1�����û���̿���������ã����Գ�ȥˮ�еij�ζ��һЩ�ܽ�����ʣ���2����ʵ������Ҫ��ȥ��Ȼˮ�еĹ������ʣ����Բ��ù��˵ķ�������3���ڼ�ͥ��ͨ��������еķ�����ʹӲˮת��Ϊ��ˮ������ˮ��Ӳ�ȣ�
�ʴ�Ϊ��
��1��������
��2�����ˣ�
��3�����
������ˮ�ľ��������ܶ࣬���ݲ�ͬ��Ҫ��ѡ��ͬ�ķ���������̿��������ˮ�еij�ζ��һЩ�ܽ�����ʣ������ܳ�ȥ��Ȼˮ�еĹ������ʣ���п���ʹӲˮת��Ϊ��ˮ������ˮ��Ӳ�ȣ�
���������⿼��ˮ�ľ����������ڼ�ͥ��ͨ��������еķ�����ʹӲˮת��Ϊ��ˮ������ˮ��Ӳ�ȣ�
�ʴ�Ϊ��
��1��������
��2�����ˣ�
��3�����
������ˮ�ľ��������ܶ࣬���ݲ�ͬ��Ҫ��ѡ��ͬ�ķ���������̿��������ˮ�еij�ζ��һЩ�ܽ�����ʣ������ܳ�ȥ��Ȼˮ�еĹ������ʣ���п���ʹӲˮת��Ϊ��ˮ������ˮ��Ӳ�ȣ�
���������⿼��ˮ�ľ����������ڼ�ͥ��ͨ��������еķ�����ʹӲˮת��Ϊ��ˮ������ˮ��Ӳ�ȣ�

��ϰ��ϵ�д�
 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
�����Ŀ
��ȥ���������е����ʣ�������Ϊ���ʣ�����ѡ���Լ�����������ȷ��һ����
| ѡ�� | ���ᴿ������ | ѡ�õ��Լ� | �������� |
| A | Na2SO4��NaOH�� | ϡ���� | �ӹ�����ϡ���ᡢ�����ᾧ |
| B | NaCl��Na2CO3�� | BaCl2��Һ | ����������BaCl2��Һ��������ϴ�ӡ����� |
| C | KCl��K2CO3�� | ϡ���� | �ӹ�����ϡHCl�������ᾧ |
| D | H2��CO2�� | ����������Һ Ũ���� | �����������ͨ������������������ Һ��ϴ��ƿ����ͨ��Ũ�����ϴ��ƿ |
- A.Na2SO4��NaOH��
- B.NaCl��Na2CO3��
- C.
KCl��K2CO3��
- D.
H2��CO2��