��Ŀ����
�����ӽǿ����ʵı仯�ǻ�ѧ���е�˼ά��ʽ����������ʾ��ͼ�ֱ��ʾ�����Ȼ��ƵIJ�ͬ��ѧ��Ӧ�������ͼʾ�ش�������⣺

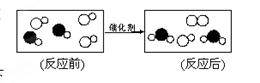
��1��ͼ1�ǽ�������������Ӧ�����Ȼ��Ƶ���ʾ��ͼ����ͼ1��֪��Ԫ�صĻ�ѧ������
������ĸ��ţ������й�ϵ��
A������������ B���ڲ���������� C�����Ӳ���
��2��ͼ2������NaOH��Һ�����ᷴӦ����ʵ�ʣ�ͼ��A��B��C��Ӧ��������ӷ��Ż�ѧʽ����Ϊ �� �� ��
��3��ͼ3��ʾ��Ӧ�Ļ�ѧ����ʽΪ ��

��1��ͼ1�ǽ�������������Ӧ�����Ȼ��Ƶ���ʾ��ͼ����ͼ1��֪��Ԫ�صĻ�ѧ������
������ĸ��ţ������й�ϵ��
A������������ B���ڲ���������� C�����Ӳ���
��2��ͼ2������NaOH��Һ�����ᷴӦ����ʵ�ʣ�ͼ��A��B��C��Ӧ��������ӷ��Ż�ѧʽ����Ϊ �� �� ��
��3��ͼ3��ʾ��Ӧ�Ļ�ѧ����ʽΪ ��
��1��A����2��OH-��H+��H2O����3��Cl2+NaClO2=ClO2+NaCl��
�����������ͼ1��֪��Ԫ�صĻ�ѧ���������������������й�ϵ������ѡA��NaOH��Һ�����ᷴӦ����ʵ����H-��H+����H2O������ͼ��A��B��C��Ӧ��������ӷ��Ż�ѧʽ����ΪOH-��H+��H2O��ͼ3��ʾ��Ӧ�Ļ�ѧ����ʽΪCl2+NaClO2=ClO2+NaCl��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
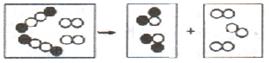
 �������� ��
�������� �� ��������Ϊһ�࣬�������п��Թ���������
��������Ϊһ�࣬�������п��Թ���������
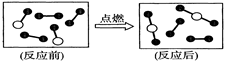
 ��
�� �ķ��Ӹ���Ϊ2:3
�ķ��Ӹ���Ϊ2:3 2CO2
2CO2 ����Ԫ�ص�ԭ�ӽṹʾ��ͼ
����Ԫ�ص�ԭ�ӽṹʾ��ͼ