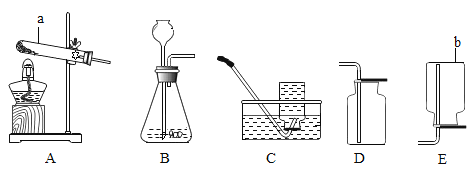
��Ŀ����
����Ŀ����ˮ�ֳ�����ˮ,��ָ��ˮ����ˮ���ʵ�������,�ﵽһ����ˮ��ָ��,������һ����Χ���ظ�ʹ�õķ�����ˮ��������������ũҵ��ˮ�ȣ�����ͼΪij��ˮ����ϵͳ����ͼ,��ش��������⡣
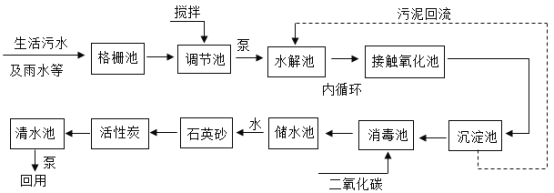
��1�����ڳ�Ԥ���������費�Ͻ���,��ʵ���������ڽ���IJ���������_____��
��2����դ��ʯӢɰ�������൱��ʵ������е�_____,����̿��������_____��
��3����������ͨ������������ȣ���ѧʽ![]() ��,����Cl�Ļ��ϼ���_____��
��,����Cl�Ļ��ϼ���_____��
��4����____�ɳ����ж���ˮ����ˮ����Ӳ��,����ˮΪӲˮ,��ʵ��������_____��
��5����Լ��ˮ��ÿ������Ӧ�����������д�ʩӦ�ᳫ����_____��
A ������ˮ���� B ����ʽ����� C ���õ�Ͱװˮֱ�ӵ���
���𰸡������� ���� ���� ![]() ����ˮ ��������ĭ�٣������� AB
����ˮ ��������ĭ�٣������� AB
��������
��1����ʵ���������ڽ���IJ��������Dz�������
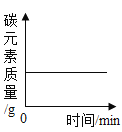
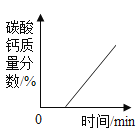
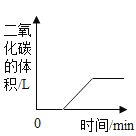
��2����դ��ʯӢɰ�ܳ�ȥ�������Ե����ʣ������൱��ʵ������еĹ��ˣ�����̿��������������
��3���ڶ�������![]() ��ѧʽ
��ѧʽ![]() ������Ԫ�صĻ��ϼ�Ϊ
������Ԫ�صĻ��ϼ�Ϊ![]() �ۣ��Ƴ��ȵĻ��ϼ���
�ۣ��Ƴ��ȵĻ��ϼ���![]() ��
��
��4���÷���ˮ�ɳ����ж���ˮ����ˮ����Ӳ�ȣ�����ˮΪӲˮ����ʵ�������Dz�������ĭ�٣������ࡣ
��5��A��������ˮ������һˮ���ã��ܽ�Լ��ˮ��Ӧ�ᳫ��
B������ʽ����֣��ܽ�Լ��ˮ��Ӧ�ᳫ��
C�����õ�Ͱװˮֱ�ӵ����������ˮ���˷ѣ���Ӧ�ᳫ��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ��С��ͬѧ�ֱ��������ſ���������ˮ�����ռ�һƿ������Ȼ�������˿ȼ�յ�ʵ�飬����ȼ������ͬ�����Ƕ���˿ȼ��������Ũ�ȵĹ�ϵ������̽����
������ʵ�飩ȡ5����ͬ����˿��ֱ��0.6mm����������ͬ������״�ֱ����������������ͬ����ƿ�н���ʵ�飬ʵ���¼����
ʵ����� | �������������% | ʵ������ |
��һ�� | 90% | ȼ�վ��ң��������䣬ȼ��ʱ�䳤��ʵ��ɹ� |
�ڶ��� | 80% | ȼ��������90%�����û�����Բ��죬ʵ��ɹ� |
������ | 70% | ȼ�ձ�80%������ȼ��ʱ���80%�Ķ̣�ʵ��ɹ� |
���Ĵ� | 60% | ȼ�ձ�70%������ȼ��ʱ����̣�ʵ��ɹ� |
����� | 50% | ��˿û��ȼ�� |
��1����˿ȼ�յ����ֱ���ʽΪ_________
��2��ͨ������̽��ʵ�飬�ɵó��Ľ���________��
��3��Ҫ�о���˿�Ĵ�ϸ��ȼ�������Ӱ�죬����ʵ���ܴﵽĿ����_______��
A ��ͬһƿ�����У��Ⱥ���в�ͬ�֡�ϸ��˿��ȼ��ʵ��
B ����ƿ��ͬŨ�ȵ������У��ֱ�ͬʱ���д֡�ϸ��˿��ȼ��ʵ��
C ����ƿ��ͬŨ�ȵ������У��ֱ���д֡�ϸ��˿��ȼ��ʵ��
����˼�����ۣ�����������Ҫ��ϸߵ�ʵ��Ӧ��ȡ_____�ռ�������
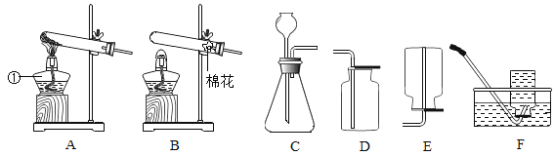
����Ŀ��ͬѧ����ѧϰ�����������ʺ�,֪������������ʹ�����ǵ�ľ����ȼ��,�ڴ˻�����,ͬѧ���������������,����һ������̽����
��1��������һ�������ǵ�ľ����ȼ�ܷ�֤�������Ǵ�����
������ʵ�飩ͬѧ�������ֻ�ʵ��̽���ǽ���ʵ��![]() ��ͼ
��ͼ![]() ,�������һϵ�����ݡ�
,�������һϵ�����ݡ�
����ƿ��� | �� | �� | �� | �� | �� |
����Ũ�ȣ���������� | 25% | 35% | 45% | �� | 65% |
������ľ����� | �� | �� | ���� | ��ȼ | ��ȼ |
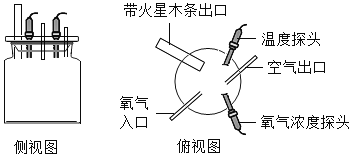
���ռ�֤�ݣ��ܺż���ƿ�ڵ�����Ũ�ȿ�����_____��
��ʵ����ۣ�_____��
����˼�����ۣ������������ݿ�֪���ռ�ƿ��Ϊ������������õ��ռ�������_____��
��2�������������˿�������е�ȼ��������Ũ�Ⱥ���˿��ϸ�й�ϵ��
ʵ���� | �� | �� | �� | �� | �� |
����Ũ�ȣ���������� | 34% | 47% | 60% | 73% | 86% |
ֱ��0.2mm��ϸ��˿ | ��ȼ�� | ����ȼ�� | ����ȼ�� | ����ȼ�� | ����ȼ�� |
ֱ��0.5mm�Ĵ���˿ | ��ȼ�� | ��ȼ�� | ��ȼ�� | ����ȼ�� | ����ȼ�� |
���ռ�֤�ݣ��ݺ�ʵ����ϸ��˿ȼ�յ�����ʵ��������_____��
��ʵ����ͣ��йط�Ӧ�Ļ�ѧ����ʽ��_____,ʵ�������,�ڼ���ƿ�ײ�������ˮ��Ŀ����_____��
��ʵ����ۣ���![]() ����Ũ��Խ��,��˿ȼ��Խ_____����_____��
����Ũ��Խ��,��˿ȼ��Խ_____����_____��
�������뽻�����������˿ȼ�յ�ʵ��ʱ,Ϊ��֤ʵ��ɹ���ע���һ��ʵ�������_____��
����Ŀ��ij�о�С�鷢�֣���һƬ����Ƭ�������������Һ�У�����������Һ�ķֽ����ʼӿ졣��С��ͬѧ����������̽����
��������⣩�����ܲ�������������ֽ�Ĵ����أ�
���������룩����������������ֽ�Ĵ�����
��ʵ����֤��
ʵ���� | ʵ����� | ʵ������ |
�� | ���Թ��м������������Һ��Ȼ�����ǵ�ľ�������Թ��� | ľ������ȼ |
�� | ��װ�й���������Һ���Թ��м���0.5 g������Ȼ�����ǵ�ľ�������Թ��� | �������������ݣ�ľ����ȼ |
�� | �����з�Ӧ���������Թ����ʣ������й��ˡ�ϴ�ӡ����ɡ����� | �Ƶù�������Ϊ0.5 g |
�� | ���������ù�������Թ��У����¼������������Һ��Ȼ�����ǵ�ľ�������Թ��� | �������������ݣ�ľ����ȼ |
���������ݡ��ó����ۣ�
��1��ʵ���֤�������ڷ�Ӧǰ��________û�з����仯��
��2��ʵ���֤�������ڷ�Ӧǰ��________û�з����仯��
���ۣ�������������������ֽ�Ĵ�����
��3��д������������ʱ��������ֽ�ķ��ű���ʽ__________��
��ʵ����չ��
��1�����о�С�������˱Ƚ�������������̵Ĵ�Ч����ʵ�飨��ͼ����ʵ��ʱ��������25 mL����Ϊ����������Ӱ��ʵ������ؾ����Բ��ơ�
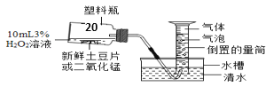
����������±���
ʵ���� | 3%����������Һ��� | ������������ | �������� |
�� | 10 mL | ������Ƭ0.5 g | a |
�� | 10 mL | ��������0.5 g | b |
������Ͳ�ռ�������ŵ�____________��
�ڸ�ʵ�黹����Ƶ�����____________��
������ʵ���е�������������ָ_________��
��2���о�С�������չ��Ѱ�ҹ�������ֽ���´��������С��������ǯ��ȡһ������ͭ˿���ŵ��ƾ��ƻ����������������ڣ�������������ͭ����Ȼ��Ѹ�ٵز���һֻװ��10 m l 3%�Ĺ���������Һ���Թ��С��۲쵽�Թ���Ѹ�ٲ����������ݡ����ǵó���������ͭ���Լӿ��������ķֽ⡣С��ͬѧ��Ϊ������۲��ɿ���ԭ����ʹ�ù�������ֽ����ʼӿ�����ػ�������_______�йء������������ʵ�鷽��__________��
��3������˵�������ᵽ��Ӱ������Ĵ�Ч����������_________��_______����֪������_______��________Ҳ���ԡ�