��Ŀ����
��������װ��ͼ��д�йؿո�

��1��װ��A��C�������٢ڵ�����______��______��
��2��ʵ������ȡ������̼�ķ���װ�ÿ�ѡ������______������ţ�װ�ã���Aװ����ȡ�����Ļ�ѧ����ʽ______��Aװ�������Ķ������Խ���______��д��Ӧ�Ļ�ѧ����ʽ����ʵ�飮
��3����ȥ���������������ʵ�ʵ�鲽�����ܽ⡢���ˡ����������õ�����װ��ijһװ���⣬����Ҫ�õ����в�����������е�______�������ʵ�飮
I��©�����ձ����������� II���ιܡ���Ͳ���Թܡ�����III������ƿ���������ƿ
��4�����ᴿ�õ����Ȼ��ƹ����ˮ����10%���Ȼ�����Һ�����Ƶõ�����Һ��������С��10%�Ŀ���ԭ��______��
��5����װ��B����25�˴���ʯ��100��ϡ������ȫ��Ӧ�����ʲ���Ӧ����ȡ������̼��װ��B������ʣ�����ʵ�����Ϊ116.2�ˣ��Լ��㣺
�ٿ��Ƶ�______Ħ��������̼��
�ڲμӷ�Ӧ��HCl�����ʵ����Ƕ��٣�������ݻ�ѧ����ʽ��ʽ���㣩
�۴���ʯ��̼��Ƶİٷֺ���Ϊ______��
�⣺��1�������Թܣ����Ƿ�Һ©����
��2����ȡ������̼��ҩƷ��ʯ��ʯ��ϡ���ᣬ���ڹ����Һ���ڳ����µķ�Ӧ�����Ӧѡ�õķ���װ����BC������Bװ�ÿ��Կ��Ʒ�Ӧ�ķ�����ֹͣ��Cװ�ÿ��Կ��Ʒ�Ӧ�����ʣ�Aװ���Ǽ��ȷ���ȡ���������Թܿ�����������Ǽ��ȸ��������ȡ��������Ӧ�ķ���ʽΪ��2KMnO4 K2MnO4+MnO2+O2����
K2MnO4+MnO2+O2����
�����ԸĶ������Խ��е�ʵ���ǣ�������ԭ����ͭ��ʵ�飬�Ķ��ķ�����ȥ����Ƥ�����ѵ����쵽�Թܵĵײ�����Ӧ�ķ���ʽΪ��H2+CuO Cu+H2O��
Cu+H2O��
��3���ܽ���Ҫ�ձ��Ͳ����������˻���Ҫ�IJ���������©������ѡ���
��4���⣺��������Ȼ������˵Ŀ����ԣ��磺����ʱҩƷ������ŷ��ˡ��ƺ����ձ�ʱ���䡢��ȡֽƬ��ҩƷû���ꡢ����ʱû��ȫ�ܽ�ȵȣ�
����ܼ�ˮ���˵�ԭ����٣�����������ȡҺ��ʱ���Ӷ�����������Һ���ձ�����ˮ�ȣ�
��5���ٷ�Ӧǰװ���ڵ����ʵ�����Ϊ25g+100g=125g����Ӧ��װ���ڵ���������Ϊ116.2g�������ɶ�����̼������Ϊ
125g-116.2g=8.8g��������̼��Ħ����Ϊ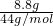 =0.2mol
=0.2mol
�ڲμӷ�Ӧ��HCl�����ʵ�����X
CaCO3+2HCl=CaCl2+CO2��+H2O
2 1
X 0.2mol
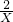 =
=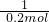
X=0.4mol
�𣺲μӷ�Ӧ���Ȼ�������ʵ���Ϊ0.4mol��
����μӷ�Ӧ��̼��Ƶ�����Ϊy
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
y 8.8g
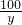 =
=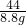 y=20g
y=20g
�����ʯ��̼��Ƶ���������Ϊ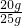 ��100%=80%
��100%=80%
�𣺴���ʯ��̼��Ƶ���������ԼΪ80%��
�ʴ�Ϊ����1���Թܣ���Һ©����
��2��B��C��2KMnO4 K2MnO4+MnO2+O2����H2+CuO
K2MnO4+MnO2+O2����H2+CuO Cu+H2O��
Cu+H2O��
��3��I
��4����ȡˮʱ���Ӷ�����ʳ�κ�����ŷ��ˡ�������Һ���ձ�����ˮ�ȣ�
��5����0.2mol��
����μӷ�Ӧ��HCl�����ʵ���Ϊx
CaCO3+2HCl=CaCl2+CO2��+H2O
2 1
X 0.2mol
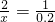
X=0.4mol
�𣺲μӷ�Ӧ���Ȼ�������ʵ���Ϊ0.4mol��
��80%
��������1���������ճ�����ѧ���������Ƽ�����;��
��2�����ݷ�Ӧ���״̬�ͷ�Ӧ����ѡ����װ�ã������ԸĶ������Խ��е�ʵ���ǣ�������ԭ����ͭ��ʵ�飬�Ķ��ķ�����ȥ����Ƥ�����ѵ����쵽�Թܵĵײ���
��3�����ݲ�����Ҫ������������
��4��������Һʱ���������õ�����Һ����������С��10%�������������Ŀ���ԭ��ɴ�����������з�����һ�������Ȼ������ˣ������ܼ�ˮ���ˣ�ץס���������棬�������ҳ������ԭ��
��5��������������Ħ�����м��㣻��ѧ����ʽ�л�ѧ������֮��Ϊ���ʵ���֮�ȣ����ڷ�Ӧ�ų����������̼�������ɷ�Ӧ������������С�����Է�Ӧǰ���������������ɶ�����̼�����������ݻ�ѧ����ʽ����òμӷ�Ӧ��̼��Ƶ������������������ʯ��̼��Ƶ�����������
���������⿼������������ȡװ�õ�ѡ���������ȡװ�õ�ѡ���뷴Ӧ���״̬�ͷ�Ӧ�������йأ�ҩƷ��ѡ��Ҳ��ҩƷ��״̬�������йأ����ݻ�ѧ����ʽ�ļ�����ۺ��Խ�ǿ��
��2����ȡ������̼��ҩƷ��ʯ��ʯ��ϡ���ᣬ���ڹ����Һ���ڳ����µķ�Ӧ�����Ӧѡ�õķ���װ����BC������Bװ�ÿ��Կ��Ʒ�Ӧ�ķ�����ֹͣ��Cװ�ÿ��Կ��Ʒ�Ӧ�����ʣ�Aװ���Ǽ��ȷ���ȡ���������Թܿ�����������Ǽ��ȸ��������ȡ��������Ӧ�ķ���ʽΪ��2KMnO4
 K2MnO4+MnO2+O2����
K2MnO4+MnO2+O2���������ԸĶ������Խ��е�ʵ���ǣ�������ԭ����ͭ��ʵ�飬�Ķ��ķ�����ȥ����Ƥ�����ѵ����쵽�Թܵĵײ�����Ӧ�ķ���ʽΪ��H2+CuO
 Cu+H2O��
Cu+H2O����3���ܽ���Ҫ�ձ��Ͳ����������˻���Ҫ�IJ���������©������ѡ���
��4���⣺��������Ȼ������˵Ŀ����ԣ��磺����ʱҩƷ������ŷ��ˡ��ƺ����ձ�ʱ���䡢��ȡֽƬ��ҩƷû���ꡢ����ʱû��ȫ�ܽ�ȵȣ�
����ܼ�ˮ���˵�ԭ����٣�����������ȡҺ��ʱ���Ӷ�����������Һ���ձ�����ˮ�ȣ�
��5���ٷ�Ӧǰװ���ڵ����ʵ�����Ϊ25g+100g=125g����Ӧ��װ���ڵ���������Ϊ116.2g�������ɶ�����̼������Ϊ
125g-116.2g=8.8g��������̼��Ħ����Ϊ
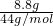 =0.2mol
=0.2mol�ڲμӷ�Ӧ��HCl�����ʵ�����X
CaCO3+2HCl=CaCl2+CO2��+H2O
2 1
X 0.2mol
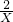 =
=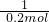
X=0.4mol
�𣺲μӷ�Ӧ���Ȼ�������ʵ���Ϊ0.4mol��
����μӷ�Ӧ��̼��Ƶ�����Ϊy
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
y 8.8g
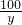 =
=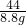 y=20g
y=20g�����ʯ��̼��Ƶ���������Ϊ
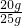 ��100%=80%
��100%=80%�𣺴���ʯ��̼��Ƶ���������ԼΪ80%��
�ʴ�Ϊ����1���Թܣ���Һ©����
��2��B��C��2KMnO4
 K2MnO4+MnO2+O2����H2+CuO
K2MnO4+MnO2+O2����H2+CuO Cu+H2O��
Cu+H2O����3��I
��4����ȡˮʱ���Ӷ�����ʳ�κ�����ŷ��ˡ�������Һ���ձ�����ˮ�ȣ�
��5����0.2mol��
����μӷ�Ӧ��HCl�����ʵ���Ϊx
CaCO3+2HCl=CaCl2+CO2��+H2O
2 1
X 0.2mol
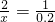
X=0.4mol
�𣺲μӷ�Ӧ���Ȼ�������ʵ���Ϊ0.4mol��
��80%
��������1���������ճ�����ѧ���������Ƽ�����;��
��2�����ݷ�Ӧ���״̬�ͷ�Ӧ����ѡ����װ�ã������ԸĶ������Խ��е�ʵ���ǣ�������ԭ����ͭ��ʵ�飬�Ķ��ķ�����ȥ����Ƥ�����ѵ����쵽�Թܵĵײ���
��3�����ݲ�����Ҫ������������
��4��������Һʱ���������õ�����Һ����������С��10%�������������Ŀ���ԭ��ɴ�����������з�����һ�������Ȼ������ˣ������ܼ�ˮ���ˣ�ץס���������棬�������ҳ������ԭ��
��5��������������Ħ�����м��㣻��ѧ����ʽ�л�ѧ������֮��Ϊ���ʵ���֮�ȣ����ڷ�Ӧ�ų����������̼�������ɷ�Ӧ������������С�����Է�Ӧǰ���������������ɶ�����̼�����������ݻ�ѧ����ʽ����òμӷ�Ӧ��̼��Ƶ������������������ʯ��̼��Ƶ�����������
���������⿼������������ȡװ�õ�ѡ���������ȡװ�õ�ѡ���뷴Ӧ���״̬�ͷ�Ӧ�������йأ�ҩƷ��ѡ��Ҳ��ҩƷ��״̬�������йأ����ݻ�ѧ����ʽ�ļ�����ۺ��Խ�ǿ��

��ϰ��ϵ�д�
 ����ѧУ�ֲ����ܲ�ϵ�д�
����ѧУ�ֲ����ܲ�ϵ�д� �ƸԺ���ȫ�����Ų��Ծ�ϵ�д�
�ƸԺ���ȫ�����Ų��Ծ�ϵ�д�
�����Ŀ




